This chapter is very important not only with the view of study but also very useful to our everyday life. It is really a homogenous mixture of two or more pure substances. This chapter is interesting and understanding. This is chapter will be beneficial for all the students who are going to participate either in 12th board exam or someone entrance exams like Neet, Jee(mains), IIT advance or for job exams. We shall provide exact notes of this chapter with the following contents.
The important contents
- Types of solution
- Concentration of solution
- Solubility and affecting factors
- Henry’s law and its application
- Raoult’s law and its application
- Ideal and non ideal solutions
- Colligative properties
- Abnormal molecular mass and vant-Hoff factor.
Solution : A homogeneous mixture of two or more non reacting substances is called solution. Ex. Sugar in water, alloys, air etc.
Types of solutions : Based on the physical state of solute and solvents
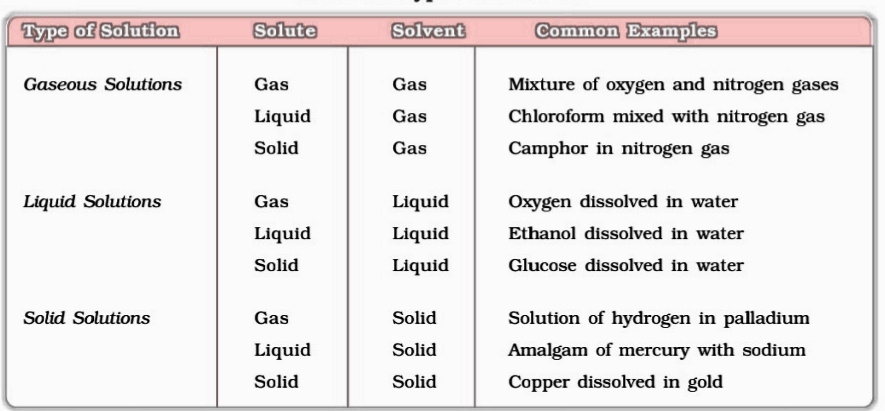
concentration of solution:
The amount of solute dissolved in the certain amount of solvent or solution is called concentration of solution. There are various ways to represent the concentration of solutions. They are followings (1) By percentage : Either in term of mass, volume or both
(a) % (w/w) = mass of any component/ mass of solution ×100
(b) % (w/v) = mass of any component/ volume of solution ×100
(c) % (v/v) = volume of any component/ volume of solution ×100
(2) By molarity : No. Of moles of solute present in per litre of solution is called molarity.
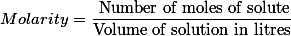
(3) By Molality: No. Of moles of solute dissolved in per kilogram of solvent is called molality. It is denoted by ‘m’. Its unit is m or mol/kg.
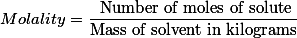
(4) By More Fraction:– The no. Of moles of any component present in per mole of solution is called mole fraction. It is denoted by X and it is dimensionless.
Let the moles of two components in a solution be n1 and n2 respectively, Then-
(5) By Normality: The no. of g-equivalent of a solute present in per litre solution is called normality. It is denoted by N.
(6) By Parts per million parts (ppm) : The mass of solute present in one million (106 ) parts by mass of solution is called ppm.
Solubility :
The concentration of a saturated solution in any terms like molarity, molality or mole fraction is called solubility of a solution.
Some important factors which affect the solubility in solid-liquid solutions:
(1) Nature of solute and solvent: Solubility in solid-liquid solution depends on the nature of of solute and solvent and acts on the principle that like dissolve like. That means polar solute is soluble in polar solvent and non polar solute is soluble in non polar solvent.
(2) Temperature: When dissolution is exothermic, solubility decreases with increase in temperature while solubility increases with increase in temperature when dissolution is endothermic. Dissolution of Ionic compounds is endothermic and dissolution of hydrated solute is exothermic.
(3) Pressure: In case of solid-liquid solution, there is no effect of pressure because solid solute are not compressible.
Some important factors which affect the solubility in Gas-liquid solutions:
(1) Nature of solute and solvents: Like solid-liquid solution, solubility acts on the principle “Like dissolves like “.
(2) Temperature: Dissolution of a gas into liquid is an exothermic process hence solubility decreases with increase in temperature.
(3) Pressure: The effects of pressure on solubility in gas-liquid solution follows Henry’s law in the following way – ” The partial pressure of any gas in a solution is directly proportional to the mole fraction of the gas.
Important characteristics of Henry’s Law Constant (KH) :
(1) Henry’s constant is a function of the nature of the gas.
(2) Greater the value of KH , lower is the solubility of the gas at the same partial pressure at a particular temperature.
(3) The value of KH increases with increase of temperature.
Application of Henry’s Law:
(1) In the application of carbonated beverages
(2) In the deep sea diving
(3) In the function of lungs
(4) For the people or climbers living at high altitude.
Vapour Pressure :
The pressure exerted by the vapours in equilibrium with the liquid/solution at a particular temperature is called vapour pressure of this liquid or solution.
The vapour pressure of a liquid depends upon the following factors:
(1) Nature of the liquid. – Weaker are the intermolecular forces, greater is vapour pressure.
(2) Temperature; – Higher is the temperature, greater is the vapour pressure.
Vapour pressure and Raoult’s Law in Liquid-liquid solution:
In a solution, the vapour pressure of any component at a given temperature is equal to the mole fraction of that component in the solution multiplied by the vapour pressure of that component in the pure state.
Let us consider, the vapour pressure of two completely miscible liquids A and B, having the mole fraction xA and xB, their partial pressure be PA and PB and the vapour pressure in pure state be PoA and PoB. Then, According to Raoult’s Law:
PA = PoA . xA and PB = PoB . xB
Raoult’s Law in special condition;
(1) when solute is volatile: When solute and solvent, both are volatile
P = (PoB – PoA)xB + PoA
(2) When solute is non volatile: – When solute is non volatile, the vapour pressure of solution decreases according to the following equation:
Ideal and Non-ideal solution:
Ideal Solution: That solution in which each component obeys Raoult’s law under all conditions of temperature and concentration is called Ideal solution.
Examples of ideal solutions:
(1) Benzene + Toluene
(2) n-Hexane + n-Heptane
(3) Chlorobenzene + Bromobenzene
Important conditions for ideal solutions:
(1) PA = PoA . xA and PB = PoB . xB (2) ∆Hmix = 0 (3) ∆Vmix = 0
Non-Ideal solution: That solution in which each component does not obey Raoult’s law under all conditions of temperature and concentration is called non- ideal solutions. Ex. Alcohol + Water, Acetone + Chloroform.
Types of non- ideal solutions: There are two types of non- ideal solutions
(1) positive deviations from ideal solutions: That solution whose vapour pressure is more than the vapour pressure as expected according to Raoult’s law is called positive deviation from ideal solutions. Ex. (1) Acetone + Ethyl alcohol (2) Acetone + Benzene (3) Ethyl alcohol +Water
Conditions for positive deviation solution: (1) PA > PoA . xA and PB > PoB . xB (2) ∆Hmix = +ve (3) ∆Vmix = +ve.
(II) Negative deviation from ideal solutions: That solution whose vapour pressure is less than the vapour pressure as expected according to Raoult’s law is called negative deviation from ideal solutions. Ex. (1) Chloroform + Benzene (2) Acetone + Aniline (3) HCl + Water
Conditions for negative deviation solution: (1) PA < PoA . xA and PB < PoB . xB (2) ∆Hmix = -ve (3) ∆Vmix = -ve.
Colligative properties: Those properties of ideal solutions which depend only on the number of particles of the solute dissolved in a definite amount of the solvent and do not depend on the nature of solute are called colligative properties.
The important colligative properties are followings:- (1) Relative lowering of vapour pressure (2) Osmotic pressure (3) Elevation in boiling point (4) Depression in freezing point
Relative lowering of vapour pressure : The ratio between the lowering in vapour pressure and the vapour pressure of solvent is called relative lowering of vapour pressure.
Osmotic pressure; The minimum external pressure applied on the surface of solution to prevent the entry of the solvent into the solution through the semipermeable membrane is called the osmotic pressure.
Expression for the osmotic pressure
This equation is called Van’t Hoff equation for dilute solutions.
Classification of solution based on osmotic pressure:
(1) Hypertonic solution- A solution which has highest osmotic pressure than any other solutions at the same condition is called hypertonic solution
(2) Hypotonic solution- A solution which has the lowest osmotic pressure than any other solutions at the same condition is called Hypotonic solution
(3) Isotonic solution- Those solutions which have same osmotic pressure at the same condition of concentration and temperature are called Isotonic solutions.
For Isotonic solutions:
Elevation in Boiling Point:
The increase in boiling point of a solution after going into solution phase from solvent phase is called elevation in boiling point. It is denoted by ∆Tb
In this case , To b < Tb Hence, ∆Tb = Tb – To b
As, Elevation in boiling point depends upon concentration of solution and it is a colligative properties hence, it can be mathematically can be expressed in following way.
∆Tb = Kb . molality
Where Kb is called the molal elevation constant. If m = 1
Then, ∆Tb = Kb
Hence molal elevation constant may be defined as the elevation in boiling point when the molality of the solution is unity.
The modified formula of Elevation in Boiling point can be written as:-
Molal elevation constant from enthalpy of vapourisation:
Depression in Freezing Point:
Decrease in Freezing Point of a liquid after going into solution phase from solvent phase is called Depression in Freezing Point. It is denoted by ∆Tf
In this case, To f > Tf Hence, ∆Tf = To f – Tf
As Depression in freezing point depends upon concentration of solution and it is a colligative properties hence, it can be expressed mathematically in following way.
∆Tf = Kf . molality
Where Kf is known as molal depression constant or cryoscopic constant. if molality(m) =1, Then ∆Tf = Kf Hence molal depression constant can be defined as the depression in freezing point when molality of the solution be unity.
The modified formula of depression in Freezing Point can be written as:-
Molal Depression Constant from enthalpy of fusion:
Abnormal molecular mass; The molecular mass of some solute in a solution determined with the help of colligative properties comes out to be different than the calculated molecular molecular mass from formula is known as abnormal molecular mass. It is denoted by Mo
To sum up, Abnormal molecular mass and calculated molecular mass can be related in the following way:
(1) When solution is ideal; Mo = Mc
(2) When solute is dissociating: Mo < Mc
(3) When solute Is associating: Mo > Mc Where Mc is calculated molecular mass.
Van’t Hoff factor:
The ratio of the experimental value of the colligative property to the calculated value of the colligative property is called Van’t Hoff factor. It is denoted by “i’
Relation between degree of dissociation and Van’t Hoff factor:
Relation between degree of association and Van’t Hoff factor:
Modified Expressions for substances undergoing association or dissociation:
Conclusion Notes of solution for chemistry
Solution is a homogeneous mixture of two or more unreacting substances. Solutions are many kinds based on the state of the solute and solvent. Any solution consists of two components as solute and solvent. The component which is maximum by amount in a solution is called solvent and the remaining are known as solutes. The amount of solutes present in the certain amount of solvent is called the concentration of the solution. Percentage by mass, molarity, molality, mole fraction , ppm are the various ways to represent the concentration of the solution. Henry’s law explains the effect of the partial pressure of the gases on the solubility of the gases in its solution. Raoult’s law explains the effect of vapour pressure on the solubility of a liquid in a solution.
FAQ Notes of solution for chemistry
Q.NO. 1 Why is molality preferred over molarity in handling a solution of chemistry?
Molality is preferred while studies are made independent of temperature. This is because molality involves masses which do not change the temperature.
Q. NO. 2 What is the effect of temperature on molarity of a solution ?
Molarity decreases because volume of the solution increase with increase in temperature but no. of moles of solute remains the same.
Q. NO. 3 Why is liquid ammonia bottle first cooled in ice before opening it ?
At room temperature, the vapour pressure of liquid ammonia is very high. On cooling, on cooling vapour pressure decreases. Hence, the liquid ammonia will not splash out.
Q. NO. 4 Why it is advised to add ethylene glycol to water in a car radiator while driving in a hill station ?
It is done to lower the freezing point of water so that it does not freeze.
Q. NO. 5 Sodium chloride or calcium chloride is used to clear snow from the roads. Why ?
Sodium chloride depresses the freezing points of water to such an extent that it cannot freeze to form ice. Hence, it melts off easily at the prevailing temperature.
