Ncert Solution Alcohol Phenol Ether: Here, we shall learn how to solve the Intext and exercise questions of alcohol, phenol and ether. This is the second chapter of organic chemistry for class 12th. We shall revise all the important topics and reactions during this way. Hence, it is essential to have a look on the notes of this chapter. We have tried our best to solve all the questions in easy and simple ways. This article will help you very much in preparation of your exams.
The main focus of Ncert Solution Alcohol Phenol Ether is to provide a good skill among the students. You can do better in your exams. You will feel interest during your exams and score a good marks without any pressure. We should try our best to solve all the questions of this chapter over and again so that we have a good hold on each and every topics, reactions and their mechanism. Let’s we have a look on the important topics of this chapter.
Learning Objectives of Ncert Solution Alcohol Phenol Ether
- To name alcohols, phenols and ethers according to IUPAC system of nomenclature.
- To discuss the reactions involved in the preparation of alcohols from alkenes, aldehydes, ketones and carboxylic acids.
- To discuss the reactions involved in the preparation of phenols from haloarenes, benzenesulphonic acids, diazonium salts and cumene.
- To discuss the reactions for the preparation of ethers from alcohols, alkyl halides and sodium alkoxides or aryloxides.
- To correlate physical properties of alcohols, phenols, and ethers with their structures.
- To discuss chemical reactions of the three classes of compounds on the basis of their functional groups.
Answers of Intext Questions in Ncert Solution Alcohol Phenol Ether
Question 1. Classify the following as primary, secondary and tertiary alcohols.
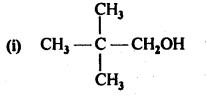
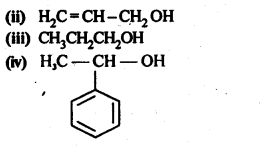
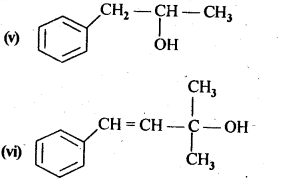
Answer: primary alcohols: (i), (ii) and (iii) Secondary alcohols: (iv) and (v). Tertiary alcohols: (vi).
Question 2. Identify allylic alcohols in the above examples. (Ncert Solution Alcohol Phenol Ether)
Answer: Allylic alcohols are (ii) and (vi).
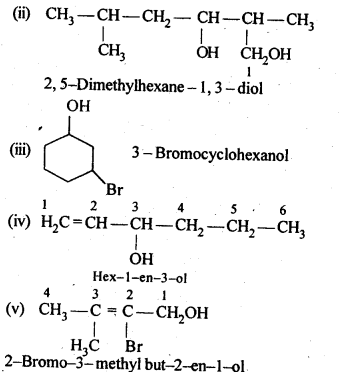
Question 3. Name the following compounds according to IUPAC system.
Answer: 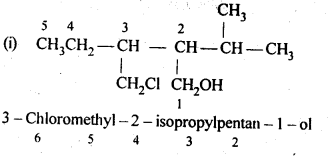
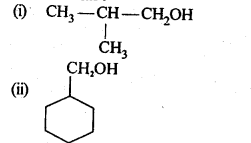
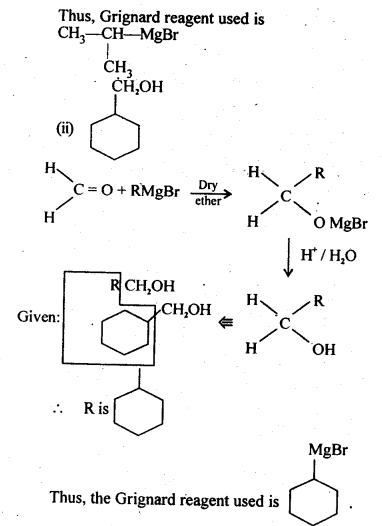
Question 4. Show how are the following alcohols prepared by the reaction of a suitable Grignard reagent on methanal ?
Answer: 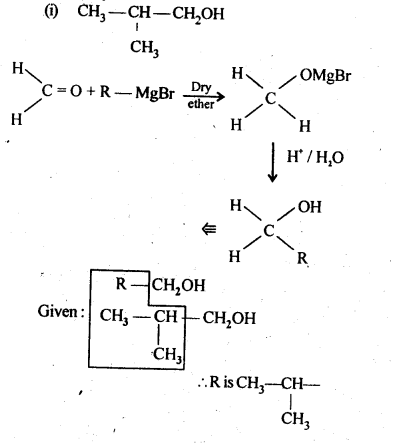
Question 5. Write structures of the products of the following reactions:(Ncert Solution Alcohol Phenol Ether)
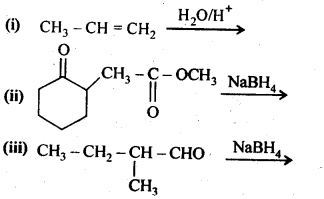
Answer: 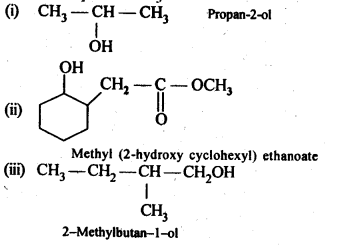
Question 6. Give structures of the products you would expect when each of the following alcohol reacts with (a)HCl-ZnCl2 (b)HBr and (c) SOCl2
(i)Butan-1-ol
(ii)2-Methylbutan-2-ol
Answer:
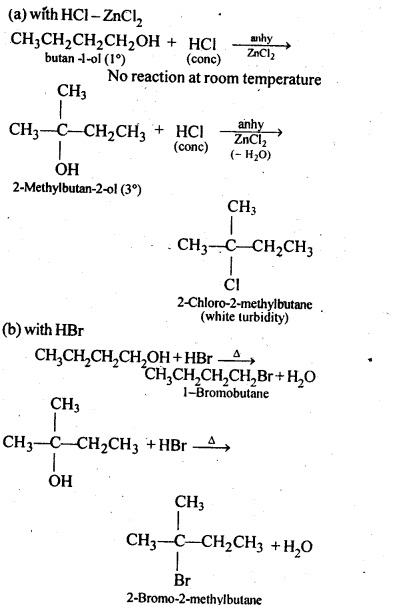
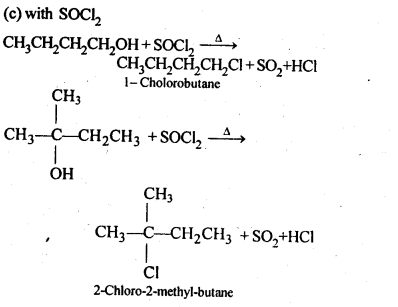
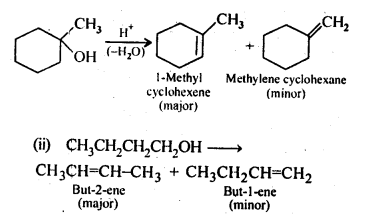
Question 7. Predict the major product of acid catalysed dehydration of
(i) 1-nicthylcyclohcxanoland
(ii) butan-1-ol (Ncert Solution Alcohol Phenol Ether)
Answer:
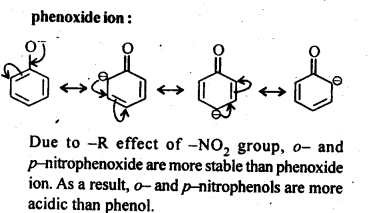
Question 8. Ortho and para nitrophenols are more acidic than phenol. Draw the resonance structures of the corresponding phenoxide ions.
Answer: The resonance structures of the corresponding phenoxide ions are followings which are obtained as intermediate during the reactions. 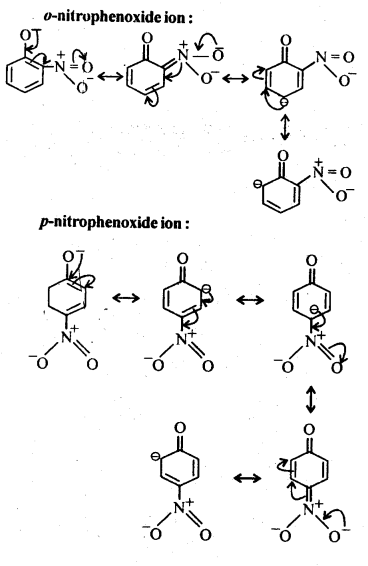
Question 9. Write the equations involved in the following reactions:
(i) Reimer-Tiemann reaction
(ii) Kolbe’s reaction (Ncert Solution Alcohol Phenol Ether)
Answer: (i) Reimer-Tiemann reaction: 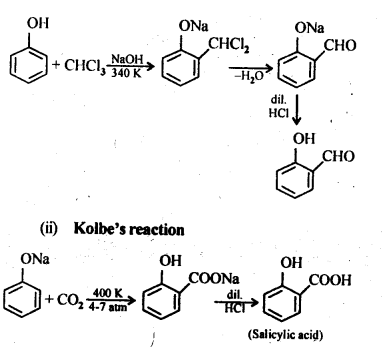
Question 10. Write the reactions of Williamson synthesis of 2-ethoxy-3-methylpentane starting from ethanol and 3-methylpentan-2-ol.
Answer: According to Williamson’s synthesis concept, the alkyl halide should be primary. Therefore, the alkyl halide should be derived from ethanol and the alkoxide ion from 3-methylpentan-2-ol. The synthesis takes place as follows: 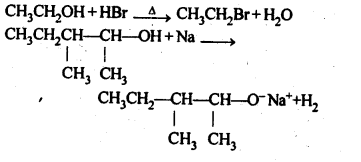
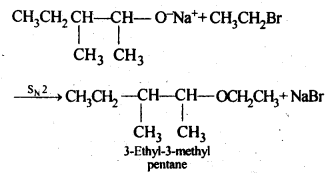
Question 11. Which of the following is an appropriate set of reactants for the preparation of l-methoxy-4- nitrobenzene and why? 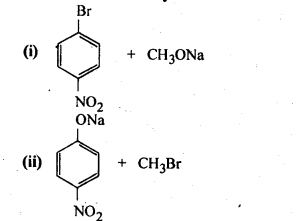
Answer: 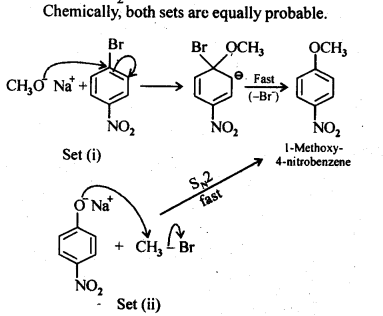
Question 12. Predict the products of the following reactions: 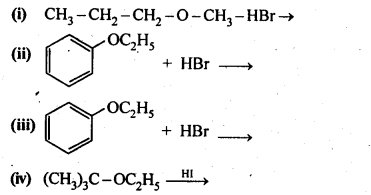
Answer: 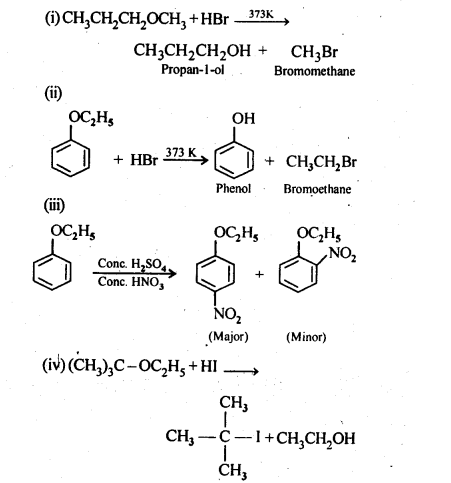
Answers of Exercise Questions in Ncert Solution Alcohol Phenol Ether
Question 1. Write IUPAC names of the following compounds: 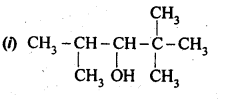
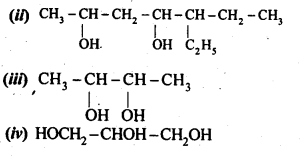
Answer: (i) 2,2,4-Trimethylpentan-3-ol
(ii) 5-Ethylheptane-2,4-dioI
(iii) Butane-2,3-diol
(iv) Propane-1,2,3-triol
(v) 2-Methylphenol
(vi) 4-Methylphenol
(vii) 2,5-DimethylphenoI
(viii) 2,6-Dimethylphenol
(ix) 1-Methoxy-2-methylpropane
(x) Ethoxybenzene
(xi) 1-Phenoxyheptane
(xii) 2-Ethoxybutane
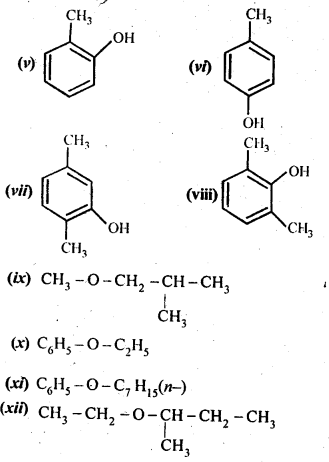
Question 2. Write structures of the compounds whose IUPAC names are as follows:
(i)2-Methylbutan-2-ol
(ii)l-Phcnylpropan-2-ol
(iii)3,5-DimethyIhexane-l,3,5-triol
(iv)2,3-Dicthylphenol
(v)1-Ethoxypropane
(vi)2-Ethoxy-3-methylpentane
(vii) Cyclohexylmethanol
(viii) 3-Cyclohexylpcntan-3-ol
(ix)Cyclopcnt-3-en-l-ol
(x)4-ChIoro-3-ethylbutan-l-ol (Ncert Solution Alcohol Phenol Ether)
Answer: 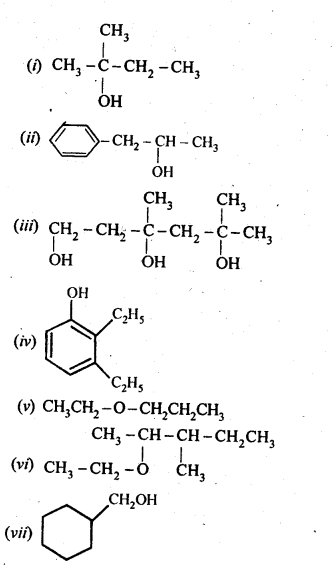
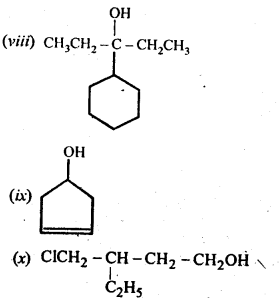
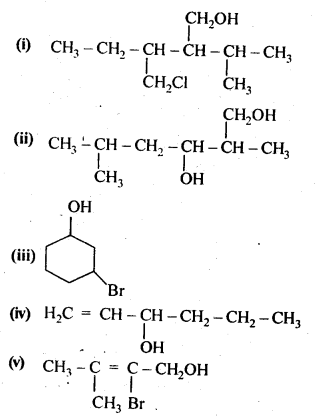
Question 3. (a) Draw the structural formulas and write IUPAC names of all the isomeric alkanols with the molecular formula C5H12O
(b) Classify the isomers of alcohols given in part (a) as primary, secondary and tertiary alcohols.
Answer: (a) The molecular formula of the given alkanols (C5H120) represents eight isomeric alkanols. They are followings:
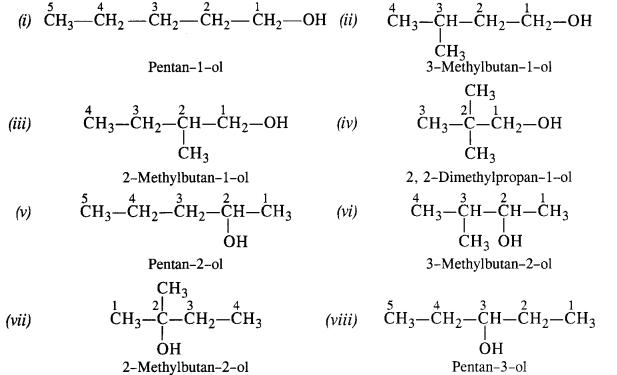
(b) Primary alcohols are (i), (ii), (iii) and (iv). Secondary alcohols are (v), (vi) and (viii). Tertiary alcohol is only (vii).
Question 4. Explain why propanol has higher boiling point than that of the hydrocarbon, butane?
Answer: The molecules of butane are joined together by weak van der Waal’s forces of attraction while the molecules of propanol are joined together by strong intermolecular hydrogen bonding. As a result propanol needs more energy to break down the force of attraction to boil. 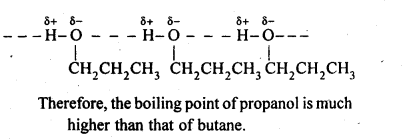
Question 5. Alcohols are comparatively more soluble in water than hydrocarbons of comparable molecular masses. Explain this fact.
Answer: Alcohol molecules have tendency to form hydrogen bond with water molecules. Therefore, alcohols are soluble in water. Hydrocarbons are non- polar and can not form hydrogen bond with water molecules hence, they are not soluble in water. 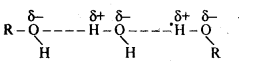
Question 6. What is meant by hydroboration-oxidation reaction? Illustrate it with an example.
Answer: Diborane reacts with alkenes as an electrophile to form trialkylboranes which upon subsequent oxidation with alkaline H2O2 give primary alcohols. This reaction is known as hydroboration- oxidation. For example: 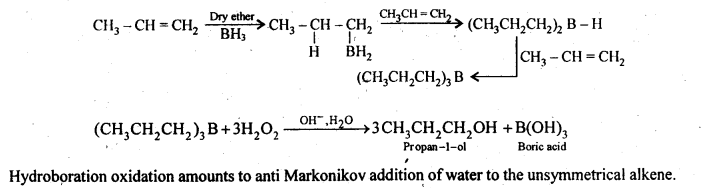
Question 7. Give the structures and IUPAC names of monohydric phenols of molecular formula, C7H8O.
Answer: The monohydric phenols of molecular formula, C7H8O are followings: 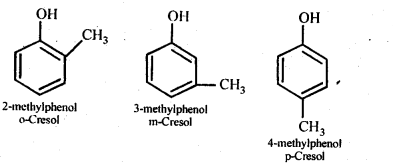
Question 8. While separating a mixture of ortho and para nitrophenols by steam distillation, name the isomer which will be steam volatile. Give reason.
Answer: o-Nitrophenol is steam volatile due to intramolecular H-bonding that is weaker in nature. Therefore, it can be separated by steam distillation. While p- Nitrophenol is not volatile because it’s molecules are hold together by inter molecular H-bond that is stronger in nature. 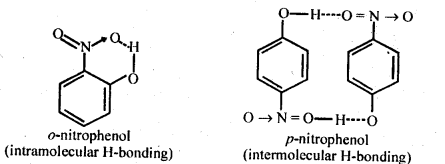
Question 9. Give the equations of the reaction for the preparation of phenol from cumene.
Answer: Phenol is prepared commercially from cumene upon aerial oxidation and subsequently hydrolysis with an aqueous acid. Acetone is produced as byproduct. Cumene is isopropyl benzene that is prepared by Friedel crafts alkylation of benzene with propene in presence of phosphoric acid. Reaction takes place in the following ways:
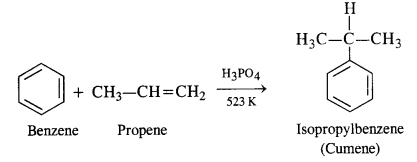
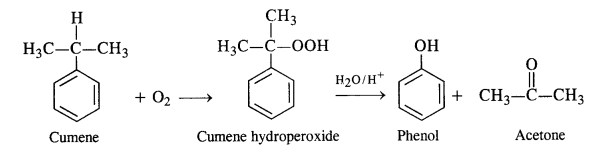
Question 10. Write chemical reaction for the preparation of phenol from chlorobenzene.
Answer: 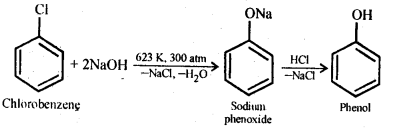
Question 11. Write the mechanism of hydration of ethene to yield ethanol.
Answer: The mechanism of hydration of ethene involves the following three steps: Step 1. Electrophilic attack by hydronium ion on alkene to give a carbocation as intermediate.
Step 2. Nucleophilic attack by water on carbocation to yield protonated alcohol.
Step 3. Deprotonation or loss of a proton to form alcohol. Reactions can be written as following: 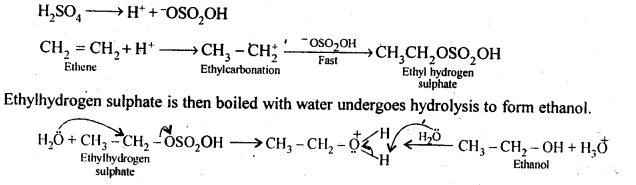
Question 12. You are given benzene, cone. H2S04and NaOH. Write the equations for the preparation of phenol using these reagents.
Answer: 
Question 13. Show how will you synthesise
(i) 1 -phenylethanol from a suitable alkene.
(ii) cyclohexylmethanol using an alkyl halide by an SN2 reaction.
(iii) Pentan-l-ol using a suitable alkyl halide?
Answer: 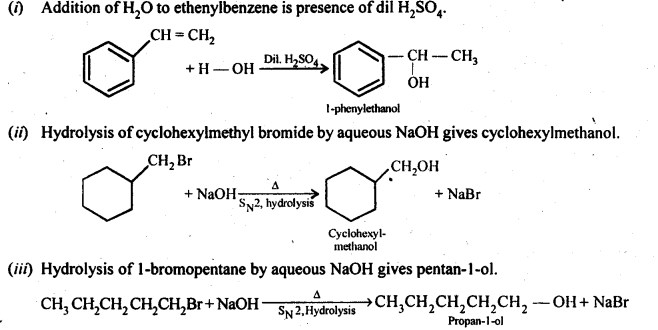
Question 14. Give two reactions that show the acidic nature of phenol. Compare its acidity with that of ethanol.
Answer: The reactions showing acidic nature of phenol are:
(a) Reaction with sodium: Phenol reacts with active metals like sodium to liberate H2, gas. 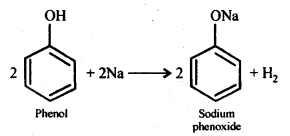
(b) Reaction with NaOH: Phenol dissolves in NaOH to form sodium phenoxide and water. 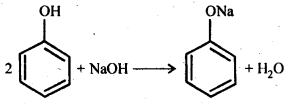
Phenol is more acidic than ethanol. Because phenoxide ion left after the loss of a proton from phenol is stabilized by resonance, while ethoxide ion left after loss of a proton from ethanol does not show any resonance. Hence it is not stable and thus, it is less acidic.
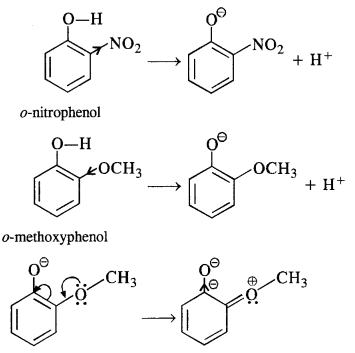
11.15. Explain why is orthonitrophenol more acidic than orthomethoxyphenol?
Answer: As we know that Nitro (-NO2) group is an electron withdrawing group while methoxy (-OCH3) group is electron releasing group in nature. The release of H+ ion is therefore, easier from o-nitrophenol while it is quite difficult from o-methoxyphenol. Apart form that, o-nitrophenoxide ion is stabilized due to resonance .
Question 16. Explain how does the – OH group attached to a carbon of benzene ring activate it towards electrophilic substitution?
Answer: Phenol may be regarded as the resonance hybrid of structures I-V, shown below. 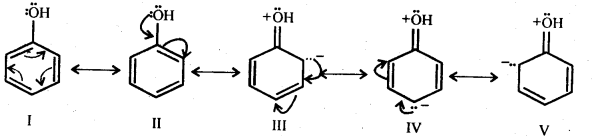
As a result of +R effect of the -OH group, the electron density in the benzene ring increases thereby facilitating the attack by an electrophile. In other words, presence of -OH group, activates the benzene ring towards electrophilic substitution reactions. Further, since the electron density is relatively higher at the two o- and one p- position, therefore electrophilic substitution occurs mainly at o- and p- positions.
Question 17. Give equations of the following reactions:
(i) Oxidation of propan-l-ol with alkaline KMnO4 solution.
(ii) Bromine in CS2 with phenol.
(iii) Dilute HNO3 acid with phenol
(iv) Treating phenol with chloroform in presence of aqueous NaOH.
Answer: 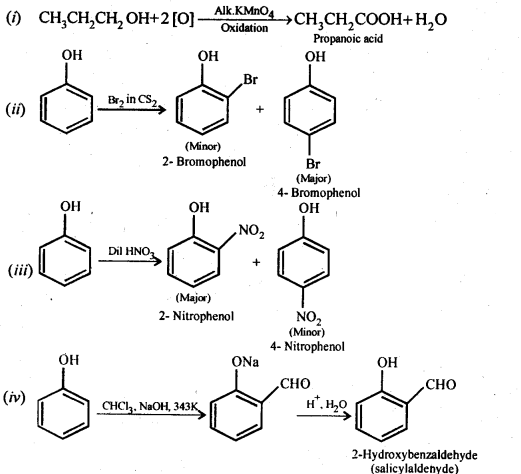
Question 18. Explain the following with an example
(i) Kolbe’s reaction
(ii) Reimer – Tiemann reaction
(iii) Williamson ether synthesis
(iv) Unsymmetrical ether
Answer: (i) Kolbe’s reaction: Sodium phenoxide when heated with C02 at 400K under a pressure of 4-7 atmospheres followed by acidification gives 2-hydroxybenzoic acid (salicylic acid) as the major product along with a small amount of 4-hydroxybenzoic acid. This reaction is called Kolbe’s reaction. 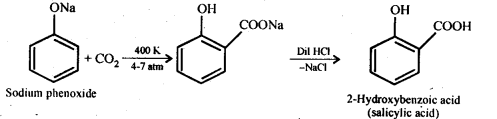
(ii) Reimer – Tiemann reaction: Treatment of phenol with CHC13 in presence of aqueous sodium or potassium hydroxide at 340 K followed by hydrolysis of the resulting product gives 2-hydroxybenzaldehyde (salicylaldehyde) as the major product. This reaction is called Reimer-Tiemann reaction. 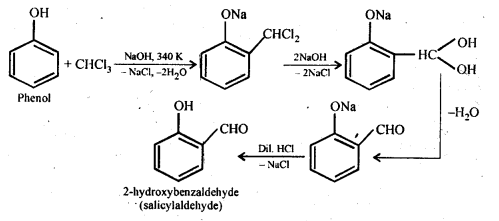
(iii) Williamson ether synthesis: It involves the treatment of an alkyl halide with a suitable sodium alkoxide to obtain ethers. The sodium alkoxide needed for the purpose is prepared by the action of sodium on a suitable alcohol. In this reaction alkyl halide should primary. Secondary and tertiary halides will predominantly give an alkene.
![]()
(iv) Unsymmetrical ether: If the alkyl or aryl groups attached to the oxygen atom are different, ethers are called unsymmetrical ethers. For example, ethyl methyl ether, methyl phenyl ether, 4-chlorophenyl- 4-nitrophenyl ether, etc.
Question 19. Write the mechanism of acid dehydration of ethanol to yield ethene.
Answer: The mechanism of dehydration of alcohols to form alkenes occur by the following three steps: 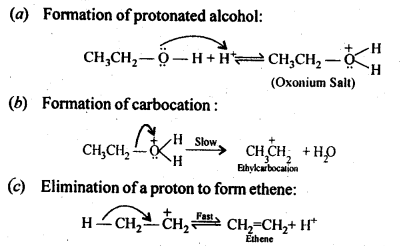
Question 20. How are the following conversions carried out?
(i) Propane → Propan-2-ol
(ii) Benzyl chloride → Benzyl alcohol
(iii) Ethyl mag. chloride → Propan-1-ol
(iv) Methyl mag. bromide → 2-Methylpropan-2-ol.
Answer: 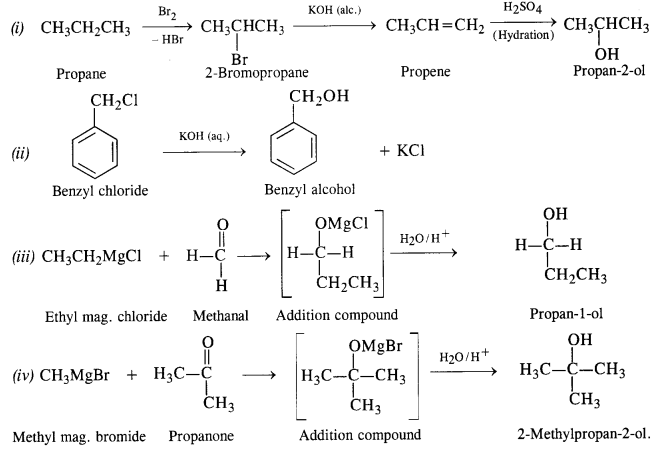
Question 21. Name the reagents used in the following reactions:
(i) Oxidation of a primary alcohol to carboxylic acid.
(ii) Oxidation of a primary alcohol to aldehyde.
(iii) Brominationofphenolto2,4,6-tribromophenol
(iv) Benzyl alcohol to benzoic acid.
(v) Dehydration of propan-2-oI to propene.
(vi) Butan-2-one to butan-2-ol .
Answer: (i) Acidified potassium dichromate or neutral/ acidic/ alkaline potassium permanganate.
(ii) Pyridinium chlorochromate (PCC), (C5H5NH)+ ClCrO3– in CH2Cl2
or Pyridinium dichromate (PDC),[(C5H5NH)2]2+Cr2O72-in CH2Cl2
(iii) Aqueous bromine, i.e., Br2/H2O.
(iv) Acidified or alkaline potassium permanganate.
(v) 85% H2S04 at 440 K.
(vi) Ni/H2 or NaBH4 or LiAlH4.
Question 22. Give reason for the higher boiling point of ethanol in comparison to methoxymethane.
Answer: Ethanol is polar and has tendency to form intermolecular Hydrogen bonding due to the presence of a hydrogen atom attached to the highly electronegative oxygen atom. As a result, ethanol exists as associated molecules. 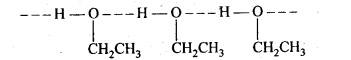
As a result, a large amount of energy is required to break the hydrogen bonds present among their molecules. Therefore, the boiling point of ethanol is higher than that of methoxymethane because it does not form any type of H-bonds.
Question 23. Give IUPAC names of the following ethers.
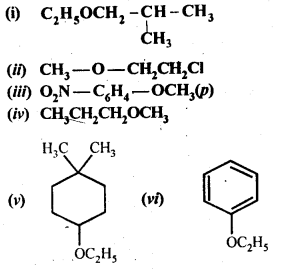
Answer: (i)1-Ethoxy-2-methylpropane
(ii) 2-Chlorlo-l-methoxy ethane
(iii) 4-Nitroanisole
(iv) 1-Methoxypropane
(v) 1 -Ethoxy-4 -4 – dimethyl cyclohexane
(vi)Ethoxybenzene
Question 24. Write the names of the reagents and equations for the preparation of the following ethers by Williamson’s synthesis :
(i) 1-Propoxypropane
(ii) 2-Methoxy-2-methylpropane
(iii) Ethoxybenzene
(iv) Methoxyethane.
Answer: 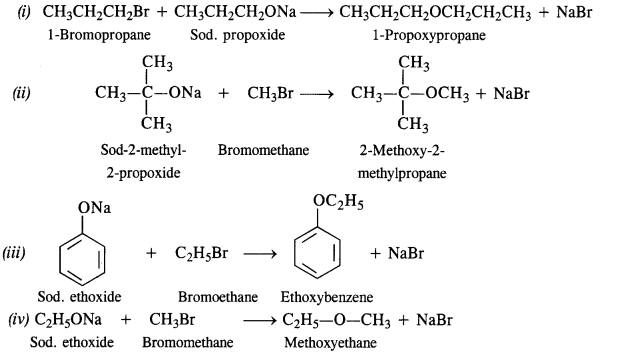
Question 25. Illustrate with examples the limitations of Willamson synthesis for the preparation of certain types of ethers.
Answer: Williamson’s synthesis is the best method for the synthesis of unsymmetrical ethers. For the synthesis of unsymmetrical ethers, a proper choice of reactants is necessary. Since Williamson’s synthesis occurs by SN2 mechanism and primary alkyl halides are most reactive in SN2 reaction, therefore, the best yields of unsymmetrical ethers are obtained when the alkyl halides are primary and the alkoxide may be primary, secondary or tertiary.
For example, tert-butylethyl ether is prepared by treating ethyl bromide with sodium tert-butoxide. 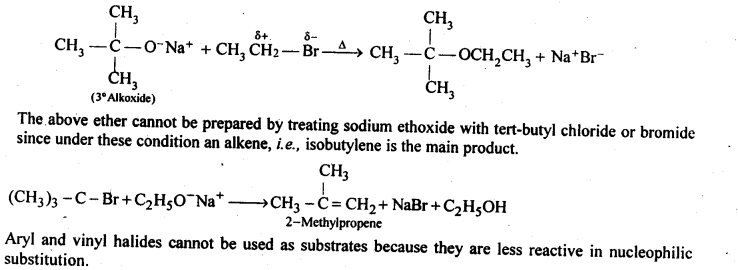
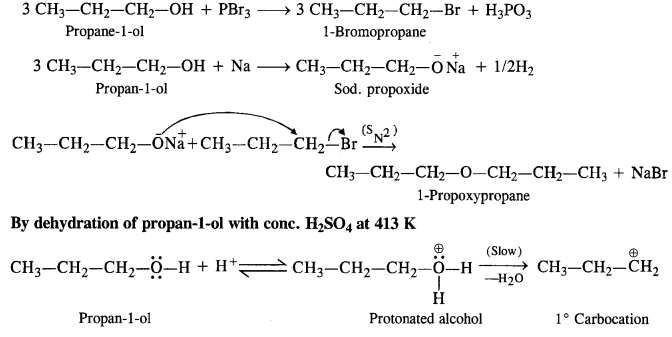
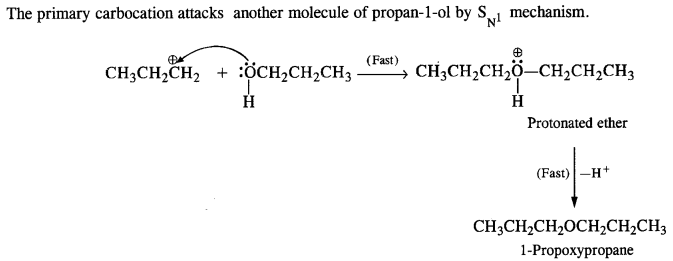
Question 26. How is 1-propoxypropane synthesised from propane-1-ol? Write mechanism of the reaction.
Answer: Two steps can be applied for the synthesis of 1-propoxypropane from propan-1-ol. In Williamson’s synthesis
The halogen derivative such as bromoderivative and sodium salt of the alcohol take part in the Williamson’s synthesis in the following ways:
Question 27. Preparation of ethers by acid dehydration of secondary or tertiary alcohols is not a suitable method. Give reason.
Answer: Acid catalysed dehydration of primary alcohols to form ethers occurs by SN2 reaction which involves nucleophilic attack by the alcohol molecule on the protonated alcohol molecule.![]()
Under the above conditions, 2° and 3° alcohols form alkenes rather than ethers. The reason behind that is steric hindrance due to which, the nucleophilic attack by the alcohol molecule on the protonated alcohol molecule does not occur. As a result protonated 2° and 3° alcohols lose a molecule of water and form stable 2° and 3° carbocation. These carbocations easily lose a proton to form alkenes and does not undergo nucleophilic attack by alcohol molecules to form ethers.
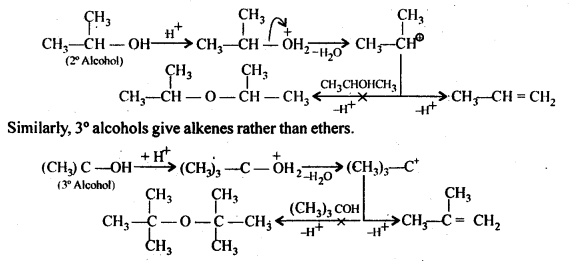
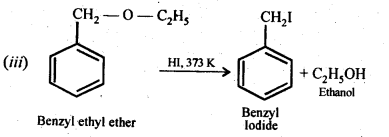
Question 28. Write the equation of the reaction of hydrogen iodide with (i)1-propoxypropane (ii)methoxybenzene, and (iii)benzyl ethyl ether
Answer: 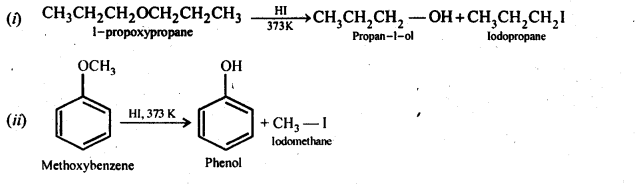
Question 29. Explain the fact that in alkyl aryl ethers, alkoxy group :
(i) activates the benzene ring towards electrophilic substitution.
(ii) directs the incoming substituents towards ortho and para positions in the ring.
Answer: (i) The alkoxy group (RO -) with lone pair of electrons on the oxygen atom activates the ortho and para positions in the ring by + M (or + R) effect as shown below : 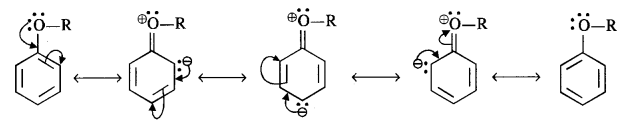
As the ortho and para positions in the benzene ring acquire high electron density and therefore electrophiles prefer to attack these positions.
(ii) The alkoxy group directs the incoming group which is electrophilic in nature towards the ortho and para positions in the ring. As a result, a mixture of isomeric products is formed.
Question 30. Write the mechanism of the reaction of HI with methoxymethane.
Answer: When equimolar amounts of HI and methoxy methane are allowed to react, a mixture of methyl alcohol and methyl iodide is formed by the following mechanism: 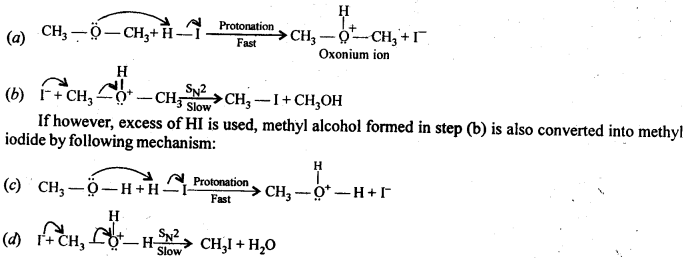
Question 31. Write equations of the following reactions:
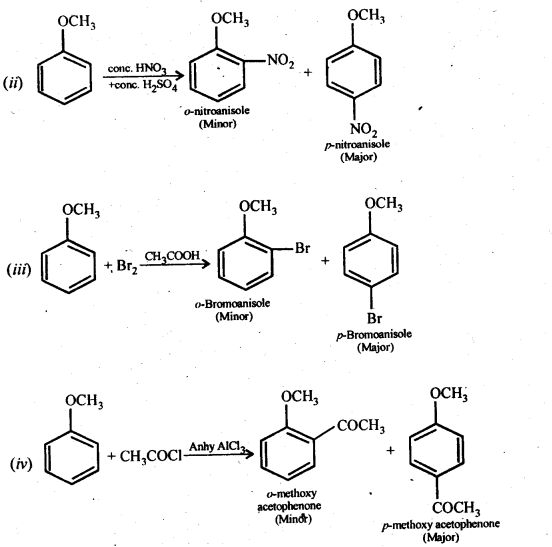
(i) Friedel-Crafts reaction -alkylation of anisole
(ii) Nitration of anisole.
(iii) Bromination of anisole in ethanoic acid medium
(iv) Friedel-Craft’s acetylation of anisole.
Answer: 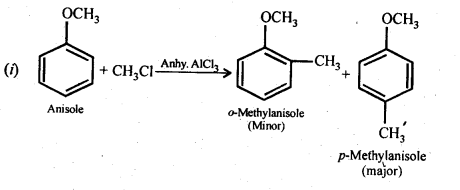
Question 32. Show how will you synthesise the following from appropriate alkenes. 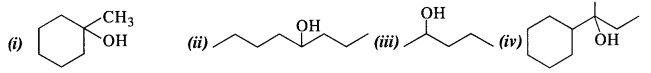
Answer: All the alcohols are formed by the hydration of alkenes in the acidic medium. The addition follows Markownikov’s rule. 1-Methylcyclohexene can be used in the reaction. 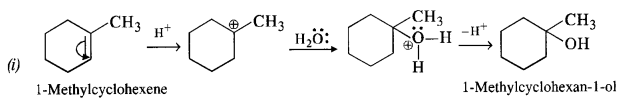
(ii) 4-Methylpent-3-ene upon hydration in the acidic medium will give the desired alcohol. 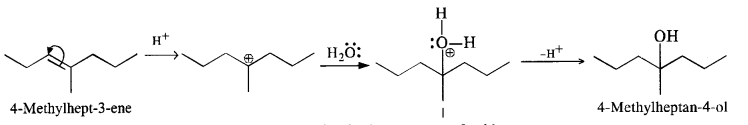
(iii) Pent-2-ene gives the desired alcohol upon hydration in the presence of acid. 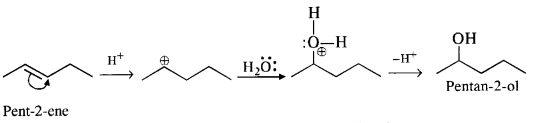
(iv) The cyclic alkene used in this reaction is 2-cyclohexylbut-2-ene. 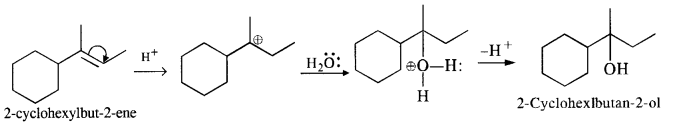
Question 33. When 3-methylbutant 2-ol is treated with HBr, the following reaction takes place: 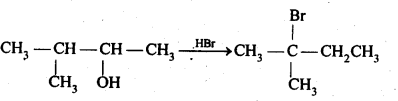 Give a mechanism for this reaction.
Give a mechanism for this reaction.
(Hint: The secondary carbocation formed in step II rearranges to a more stable tertiary carbocation by a hydride ion shift from 3rd carbon atom.)
Answer: 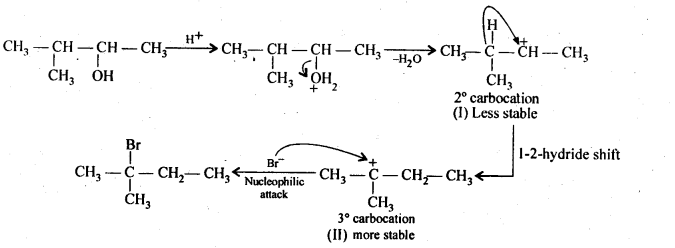
Protonation of the given alcohol followed by loss of water gives a 2° carbocation(I), which being unstable rearranges by 1,2-hydride shift to form the more stable 3° carbocation (II). Nucleophilic attack by Br– ion on this carbocation (II) gives the final product as 2-Bromo – 2- methyl propane.

