Alcohol, Phenol and Ether is the 2nd most important chapter of organic chemistry for 12th. We shall learn about their kinds, preparation, uses and properties with suitable examples. As we know that alcohol, Phenol and Ether have multi purpose uses in our daily life. This chapter is also very important for the preparation of 12th board exam along with Jee(mains), IIT advance and neet exams. Besides these faculties, questions are also arisen in other competitive exams like NDA, BPSC, UPSC, Railways and SSC exams. We have done our best to include all the necessary topics in this article so that no one return empty hand after reading this article. Let’s start this article.
What are Alcohol, Phenol and Ether?
Alcohol. The hydroxy derivatives of aliphatic, alicyclic or alkyl parts of benzene are generally known as alcohol. For example CH3CH2OH (ethyl alcohol ), C6H5CH2OH (Benzyl alcohol).
Phenol. The hydroxy derivatives of benzene are called Phenol. For example C6H5OH.
Ether. The alkoxy or aryloxy derivatives of any hydrocarbons are called ether. For example Dimethyl ether (CH3OCH3), (methyl phenyl ether)C6H5OCH3.
Classification of Alcohol, Phenol and ether
1. Based on the number of hydroxyl group, alcohols and phenols may be mono-, di-, tri- or polyhydric compounds depends on they have one, two, three or more hydroxyl groups.
2. Based on the hybridisation of carbon atoms through which -OH group is attached, monohydric alcohols are classified in the following way:
a. Compounds containing Csp3 – OH bond. This type of compounds are mainly alcohols. They are further subdivided as primary, secondary and tertiary alcohols. For example alkylic alcohols, allylic alcohols and benzyl alcohols.
b. Compounds containing Csp2 – OH bond. This type of composition is found in vinyl alcohols and phenols.
3. Ethers have been classified as simple or mixed ethers. If same hydrocarbon chains are linked both side of oxygen, it is called simple or symmetrical ether. While different types or size of hydrocarbons are linked either side of oxygen, it is called mixed or unsymmetrical ether.
Preparation of alcohols
Alcohols are prepared by the following methods.
1. From alkenes by acid catalysed dehydration. Alkenes react water in the presence of acid as catalyst to form alcohol. There is addition of water. In case of unsymmetrical alkenes, the addition of water takes place according to Markovnikov’s rule.
2. By hydroboration oxidation of alkenes. Diborane (BH3)2 reacts with alkene to give trialkyl borane as addition product. This is oxidised to alcohol by hydrogen peroxide in the presence of aqueous sodium hydroxide.
3. By reduction of aldehydes and ketones. Aldehydes and ketones can be reduced to the primary and secondary alcohols having same carbon atoms in the presence of catalyst. The usual catalyst is a finely divided metals such as platinum, palladium or nickel. Reduction of aldehydes and ketones can be also done in the presence of reducing agents NaBH4 or LiAlH4.
4. By reduction of Carboxylic acid and esters. Carboxylic acids are reduced to primary alcohols by lithium aluminium hydride in excellent yields but it is very expensive. Hence Carboxylic acid at first converted into ester then reduced to alcohols by catalytic hydrogenation.
5. From Grignard reagents. Aldehydes or ketones are treated with Grignard reagent to form addition product which further on hydrolysis gives alcohols.
Preparation of Phenol
1. From Haloarenes. Chlorobenzene is fused with NaOH at 623K and 323 atmospheric pressure to give Phenol. It is also known as carbolic acid.
2. From Benzenesulphonic acid. Benzene is sulphonated with oleum to get Benzenesulphonic acid. Now it is converted into sodium peroxide on heating with molten sodium hydroxide. Acidification of this sodium salt gives phenol.
3. From diazonium salts. A diazonium salt is formed by treating an aromatic primary amine with nitrous acid (NaNO2 + HCl) at 278K. Now diazonium salts are hydrolysed to Phenol by warming with water.
4. From Cumene (isopropylbenze). Cumene is oxidised in the presence of air into cumene hydro peroxide. Now it is converted into Phenol and acetone by treating with dilute acid.
Physical properties of Alcohols and Phenols
1. Boiling point. The boiling point of alcohols and Phenols increases with increase in the number of carbon atoms. In case of alcohols. Their boiling points decreases with increase of branching in carbon chain. The boiling point of alcohols and Phenols are higher in comparison to hydrocarbons, ethers, having comparable molecular masses. The high boiling point of alcohols is due to the formation of Intermolecular hydrogen bonding.
2. Solubility. Solubility of alcohols and Phenols in water is due to their ability to form hydrogen bond with water molecules. Their solubility decreases with increase in the size of alkyl or aryl groups.
Chemical properties of alcohols and Phenols.
Alcohols are versatile compounds. They behave both like nucleophile and electrophile. When the bond between O-H is broken, alcohols react like nucleophile. While when the bond between C – OH is broken, they behave like electrophile.
1. Reactions involving cleavage of O – H bond:
A. Acidity of alcohols and Phenols:
(I) Alcohols and Phenols react with active metals such as sodium, potassium and aluminium to give corresponding alkoxides/ phenoxides and hydrogen. In addition to this Phenol reacts with aqueous sodium hydroxide to form sodium phenoxides. These reactions justify that alcohols and Phenols are acidic in nature.
(II) Acidity of alcohols and Phenols. An electron releasing group decreases the acidic strength of alcohols and Phenols. These groups are CH3-, C2H5– etc. Whereas electron withdrawing groups increase the acidic strength of alcohols and Phenols. These groups are Cl-, NO2-, CN- etc.
⇒ We should note that Phenols are more acidic than alcohol because phenoxides ion shows resonance structures more than Phenol but not so happens in case of alcohols.
(III) Esterification. Alcohols and Phenols react with carboxylic acids, acid chloride and acid anhydride to form ester and this process is called esterification. For example ethyl alcohol reacts with acetic acid to form ethyl acetate. Aspirin is also the product of esterification in which salicylic acid reacts with acetic anhydride.
2. Reactions involving cleavage of C – OH bond.
This type of cleavage takes place only in alcohols. Alcohols react with hydrogen halide or phosphorus tri halide to form alkyl halides. Phenols show this type of reaction only with zinc.
3. Dehydration. Alcohols undergo dehydration to form alkanes on treating with protic acid like conc. H2SO4 or H3PO4, or catalyst such as anhydrous ZnCl2 or alumina. For example ethyl alcohol undergoes dehydration on heating with conc H2SO4 at 443K temperature to give ethene.
C2H5OH → CH2 = CH2 + H2O
The relative ease of dehydration of alcohols follow the following order
Tertiary > Secondary > primary
4. Oxidation. Strong oxidising agent such as acidified KMnO4 are used for getting Carboxylic acid directly from alcohols. CrO3 in anhydrous medium is used as oxidising agent for the isolation of aldehyde from primary alcohols. However a better reagent for oxidation of primary alcohols to aldehyde is pyridinium chlorochromate (PCC). Secondary alcohols are oxidised to ketones by chromic anhydride (CrO3). Tertiary alcohols do not undergo oxidation reaction.
5. Heating with copper. When the vapours of a primary or secondary alcohols are passed over heated at 573K, dehydrogenation takes place and an aldehyde or ketone is formed. While tertiary alcohols undergo dehydration to form alkenes.
Reactions of Phenol
1. Electrophilic substitution reactions.
(I) Nitraton: With dilute Nitric acid at low temperature, Phenols produce ortho and para nitro Phenols. With conc Nitric acid, it produces 2, 4, 6 – trinitrophenol.
(II) Bromination. On treating Phenol with bromine in low polarity of solvents like CHCl3 or CS2 at low temperature, it produces ortho and para bromophenol. In which para bromophenol is maximum. When Phenol is treated with bromine water, a white precipitate of 2, 4, 6 – tribromophenol is obtained.
2. Kolbe’s reaction. Phenoxide ion generated by treating Phenol with sodium hydroxide undergoes electrophilic substitution with a weak electrophile CO2, ortho hydroxy benzoic acid or salicylic acid is formed as the main product.
3. Reimer – Tiemann reaction. On treating Phenol with Chloroform in the presence of sodium hydroxide, a – CHO group is introduced at the ortho position of benzene ring, salicylaldehyde is formed and this reaction is called Reimer – Tiemann reaction.
4. Reaction with Zinc dust. Phenol is converted to benzene on heating with zinc dust.
5. Oxidation of Phenol. Oxidation of Phenol with chromic acid or Na2Cr2O7 with H2SO4 produces a conjugated diketone known as benzoquinone.
Preparation of Ether.
1. By dehydration of alcohols. Alcohols undergo dehydration in the presence of protic acid like H2SO4 or H3PO4. The formation of the reaction product, alkene or ether depends on the condition of the reaction. If process is done at 413K, ether is the main product but at 443K, alkene is the main product. We should also know that the formation of ether by this process is nucleophilic bimolecular reaction. This method is suitable for the ether having primary alkyl group only.
R – OH + R – OH → R – O – R + H2O
2. By Williamson synthesis. It is an important laboratory method for the preparation of symmetrical and unsymmetrical ethers. In this method an alkyl halide is reacted with Sodium alkoxides. This reaction involves SN2 attack of an alkoxide ion on primary alkyl halide.
RX + R’ONa → R – O – R’ + NaX
Physical properties of ethers
Boiling points. Ethers are less polar covalent compounds hence their boiling points are same as of alkanes having comparable molecular masses. Their boiling point increase with increase in molecular masses.
Solubility. The miscibility of ethers with water resemble to those of alcohols having same molecular masses. Because oxygen of ether can also form H – bond with water like alcohols.
Chemical properties of ethers
1. Due to cleavage of C – O bond:
Ethers are least reactive but under drastic conditions with excess of hydrogen halide mostly HBr or HI, the cleavage of C – O bond in ether takes place to form two alkyl halides.
R – O – R’ + HX → R – X + R’ – OH
R’ – OH + HX → R – X + H2O
The order of reactivity of hydrogen halide is as follows:
HI > HBr > HCl > HF
2. Electrophilic substitution reactions.
(I) Halogenation. When anisole is treated with bromine in the presence of ethanoic acid, it forms p- Bromoanisole as major product and o- Bromoanisole as minor product.
2. Nitraton. Anisole reacts with conc H2SO4 and HNO3 to produce p- Nitroanisole as major product and o- Nitroanisole as minor product.
3. Friedel crafts reactions. Anisole undergoes Friedel crafts reactions. The alkyl or acyl group is introduced at ortho or para position of anisole in the presence of anhydrous aluminium chloride. Reactions are following.
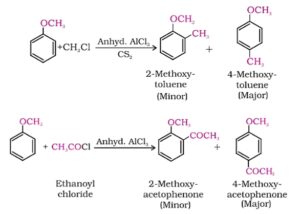
Summary of Alcohol, Phenol and Ether
In this notes of Alcohol, Phenol and Ether, we have learnt about their classification very well. We have also discussed their definition. The physical and chemical properties of Alcohol, Phenol and Ether were discussed properly. We have explained their preparation with suitable examples. We hope that this notes will be very helpful in your goal. If you like this notes, please share among your friends and favorites.
FAQ in Alcohol, Phenol and Ether
Q.No 1. What is rectified spirit ?
Ans. 95% ethyl alcohol is called rectified spirit.
Q.No 2. What is absolute alcohol ?
Ans. 100% ethyl alcohol is called absolute alcohol.
Q.No 3. What is power alcohol ?
Ans. A mixture of absolute alcohol (20%), petrol (80%) and some benzene as a cosolvent.
Q.No 4. Phenol is an acid but does not reacts with sodium bicarbonate solution why ?
Ans. Phenol is a weaker acid than carbonic acid (H2CO3) and hence does not liberate CO2 from sodium bicarbonate.
Q.No 5. Why are Grignard reagents soluble in ether but not to benzene?
Ans. Grignard reagents form coordination complexes with ether but not with benzene since the former has lone pair of electrons but the latter does not.