Aldehydes Ketones and Carboxylic Acid is very important chapter of organic chemistry for class 12th. In this notes we shall read about the definition of Aldehydes Ketones and Carboxylic Acid. We shall also learn about nomenclature, preparation, properties and uses of these compounds in details. This chapter is very useful and necessary for the students who are preparing for the entrance exams of medical and engineering. We have included all the necessary topics of this chapter which will help to solve all the questions to be asked not only in the entrance exams but also in central and state level competitive exams. The language of this notes is simple and easy.
What are Aldehydes Ketones and Carboxylic Acid?
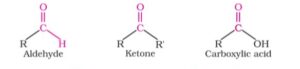
Aldehydes, Ketones, and Carboxylic Acids are known as carbonyl compounds in which carbon and oxygen atom are linked together by double bond. In aldehyde, one hydrogen atom is attached to the carbonyl carbon atom. In ketone, two carbon groups are attached to the carbonyl carbon atom and in carboxylic acids a hydroxyl group is attached to the carbonyl carbon atom. These organic compounds play very important role in the field of organic chemistry and also have many industrial applications.
What are Aldehydes?
Those organic compounds which have the functional group -CHO are called aldehydes. In this compound doubly bonded carbonyl carbon is attached to the R group and a hydrogen atom. Where R stands for alkyl group or aryl group.
Preparation of Aldehydes
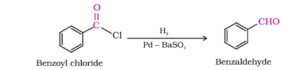
1. When acid chlorides are reduced by hydrogen in the presence of palladium as catalyst spread on barium sulfate. Aldehyde is formed. This reaction is known as Rosenmund reduction. This reaction is generally used for the preparation of aromatic aldehydes. ketones and formaldehyde can not be prepared by this method.
2. Nitriles are reduced to imine with stannous chloride in the presence of hydrochloric acid which on hydrolysis give corresponding aldehyde. This reaction is called Stephen reaction.
RCN + SnCl2 + HCl —— RCH = NH
RCH = NH + H2O—— RCHO + NH3
Properties of Aldehydes
1. Methanal or formaldehyde is a gas at room temperature. Ethanal is a volatile liquid. other aldehydes are either liquid or solid at room temperature.
2. The boiling point of aldehydes are higher than ethers and hydrocarbons having comparable molecular masses.
3. The lower members of aldehydes like methanal and ethanal are soluble in water. With the increase in their molecular masses, their solubility decreases.
4. Aldehydes are some what polar in nature. They are involved in nucleophilic addition reactions.
5. Aldehydes undergo reduction to form primary alcohols in presence of LiAlH4, NaBH4 or H2 in presence of Pt or Ni. They can be also reduced to corresponding alkanes by Clemmenson or Wolfkishner reduction.
6. Aldehydes can be oxidised into Carboxylic acid in presence of oxidising agents like K2Cr2O7, KMnO4, HNO3.
7. Aldehydes containing α- acidic hydrogen atom undergo aldol condensation to form β- hydroxy aldehyde in the presence of dilute alkalies.
8. The aldehydes which do not have α- acidic hydrogen atom, undergo self oxidation and reduction in the presence of concentrated alkali to form corresponding alcohol and Carboxylic salt. This reaction is known as Cannizzaro reaction.
9. Aromatic aldehyde involves in electrophilic substitution reactions and produces m- directing products.
Nomenclature of Aldehydes
In Acyclic and Aliphatic Aldehydes, the suffix “-al” is used after their longest carbon chain as word root. For example, CH3CH2CH2CH2CHO is called pentanal since it has five carbons in the chain.
When the aldehyde functional group is linked to a ring, “carbaldehyde” must be used as the suffix . For example, C6H11CHO is called cyclohexanecarbaldehyde. When -CHO group is not the principal functional group, the prefix formyl is used to highlight this group. For example CH3CH(CHO)CH2COOH is named as 3- Formylbutanoic acid.
| Formula | IUPAC name | Common name |
| HCHO | Methanal | Formaldehyde |
| CH3CHO | Ethanal | Acetaldehyde |
| CH3CH2CHO | Propanal | Propionaldehyde |
Uses of Aldehydes
- In chemical industries, aldehydes are used as starting materials and reagents for the synthesis of other products.
- Formaldehyde is well known as formalin (40%) solution used to prepare biological specimen and bakelite.
- Acetaldehyde is used in silvering of mirrors.
- Paraldehyde is used in medicine as a hypnotic.
- Benzaldehyde is used as a flavouring agent in perfumery industry and in the manufacture of dyes like malachite green.
What are Ketones?
The carbonyl compounds in which the carbon of carbonyl group is attached with two same or different carbon chains are called ketones. For example diethyl ketone, ethyl methyl ketone.
Types of ketones. There are two types of ketones as simple ketones and mixed ketones.
Preparation of Ketones?
1. From acyl chloride
Treatment of acyl chloride with dialkylcadmium, prepared by the reaction of cadmium chloride with Grignard reagent, gives ketones.
2RMgX + CdCl2 → R2Cd + 2Mg(X)Cl
2R’COCl + R2Cd → 2R’COR + CdCl2
2. From nitriles
Treating a nitrile with Grignard reagent followed by hydrolysis gives ketone.
3. From benzene or substituted benzene
When benzene or substituted benzene is treated with acid chloride in the presence of anhydrous aluminium chloride, it gives ketone. This reaction is also known as Friedel crafts acylation.
Properties of ketones?
1. Ketones are liquid or solid at room temperature.
2. The boiling point of ketones are higher than hydrocarbons, ethers and even aldehydes due to being much polar.
3. The lower members of ketones like propanone are soluble in water with all proportion due to forming hydrogen bond with water molecules. Their solubility in water decreases with increase in molecular masses.
4. Like aldehydes, ketones also involve in nucleophilic addition reactions.
5. Ketones after reduction by reducing agents like H2 in presence of Pt or Pd, LiAlH4 or NaBH4 give secondary alcohols.
6. Ketones can be reduced to alkanes by Clemmenson reduction or Wolff kishner reduction.
7. Ketones are generally oxidised under vigorous conditions that means by strong oxidising agents and at elevated temperature to give a mixture of carboxylic acids.
8. Ketones having α- acidic hydrogen atoms, undergo aldol condensation and produce β- hydroxy ketones.
9. Aromatic ketones undergo electrophilic substitution reactions and produce m- directing compounds.
10. Ketones don’t involve in Cannizzaro reactions.
Nomenclature of Ketones
In naming of ketones by IUPAC system, the “one” suffix is applied at the end after the name of hydrocarbons. For example CH3COCH3 is written as propanone. When carbonyl group is assigned as side chain, it is named as keto or oxo. For example CH3COCH2CHO is named in IUPAC system as 3-oxobutanal. In common system, name of carbon chains present before and after the carbonyl group as substitutes are written in alphabetical order before ketone. For example CH3COC2H5 is named as ethyl methyl ketone.
Uses of ketones
1. Acetone and ethyl methyl ketones are widely used as solvents in industry.
2. Acetone is also one of the constituent of liquid nail polish.
3. Acetone is used to prepare a number of chemicals such as Chloroform, ketene, acetic anhydride etc.
4. Ketones are used for fragrance and as flavouring agents.
What are Carboxylic acid ?
That carbonyl compound in which sp2 hybridised carbon of carbonyl group is linked with hydrogen or carbon chain in one side and by hydroxyl group on other side is called Carboxylic acid. The functional group of this compound is -COOH.
Preparation of Carboxylic acid ?
Some important methods for the preparation of Carboxylic acids are followings:
1. From primary alcohols and aldehydes.
Primary alcohols are readily oxidised to carboxylic acids with common oxidising agents such as KMnO4 in neutral, alkaline and acidic medium and K2Cr2O7 or CrO3 in acidic medium. Aldehydes are also oxidised into carboxylic acids by mild oxidising agent.
2. From nitriles and amides
Nitriles are hydrolysed to amides and then to carboxylic acids in the presence of H+ or OH– as catalyst.
3. From Grignard reagents.
Grignard reagents react with CO2(s) to form salts of carboxylic acids which give corresponding carboxylic acids after acidification with mineral acids.
Properties of Carboxylic acids ?
1. Aliphatic carboxylic acids up to nine carbon atoms are colourless liquids at room temperature with unpleasant odour.
2. The higher acids are wax like solids and are odourless due to low volatility.
3. The boiling point of carboxylic acids are higher than hydrocarbons, ether, aldehyde, ketones and even from alcohols having comparable molecular masses due to have tendency to form more extensive hydrogen bond.
4. Simple aliphatic carboxylic acids having up to four carbon atoms are miscible in water due to the formation of hydrogen bond with water molecules. Their solubility decreases with increase in the size of carbon chains.
5. The carboxylic acids evolve hydrogen gas with electropositive metals and alkalies to form salts similar to Phenol.
6. Carboxylic acids on heating with mineral acids such as H2SO4 or P2O5 give corresponding anhydride
7. Carboxylic acids react with alcohol or Phenol in the presence of mineral acids such as concentrated H2SO4 or HCl gas to form ester.
8. Carboxylic acids are reduced to primary alcohols lithium aluminium hydride or better with Diborane.
9. Carboxylic acids lose carbon dioxide to form hydrocarbons when its sodium salts are treated with soda lime. This process is called decarboxylation.
10. Carboxylic acids having an a- hydrogen are halogenated at a- position on treatment with chlorine or bromine in the presence of the small amount of red Phosphorous to give a- halocarboxylic acid. This process is known as Hell- volhard Zelinsky reaction.
Nomenclature of Carboxylic acids
In IUPAC system, suffix “oic acid ” is applied after the name of carbon chain. For example CH3COOH is named as ethanoic acid. In some cases, when the carbon atom of -COOH group is not included in parent chain carboxylic acid is used as suffix. This happens when an aliphatic compounds contain three or more -COOH groups in parent chain or hydrocarbon chain is alicyclic. Common name is based on the source from which they are obtained. For example HCOOH is named as formic acid obtained from red ants (Latin name Formica)
Uses of Carboxylic acids
1. Formic acid is as a coagulating agent for latex in rubber industry.
2. Ethanoic acid is widely used as vinegar in food industry.
3. Sodium benzoate is used as a preservative for tomato sauce, fruit jams and juices in food industry.
4. Higher fatty acids are used for the manufacture of soaps and detergents.
5. Adipic acid is used in the manufacture of nylon-66.
Conclusion
Aldehydes Ketones and Carboxylic Acid are the members of carbonyl family. In these organic compounds divalent carbon of carbonyl group is attached by different chain of carbon atoms and/ or hydrogen or hydroxyl group. We have discussed well about the preparation, uses, classification, properties and their nomenclature both by common and IUPAC system. We hope that this notes will help you very much and feel easy to learn this chapter. Therefore, we shall request you to share this notes among your friends and favourites.
FAQ for Aldehydes Ketones and Carboxylic Acid
Q.No 1. What is vinegar ?
Ans:- An 8-10% solution of acetic acid in water is called vinegar.
Q.No 2. Why are carboxylic acids called fatty acids?
Ans:- Since the higher acids were first obtained by saponification of oils and fats.
Q.No 3. What is Fehling solution?
Ans:- Alkaline solution of copper sulphate containing Rochelle salt is called Fehling solution.
Q.No 4. What makes acetic acid a stronger acid than Phenol ?
Ans:- Resonance stabilization of acetate ion is greater than that of phenoxide ion.
Q.No 5. Which type of aldehydes undergo Cannizzaro reaction?
Ans:- Aliphatic and aromatic aldehydes which do not contain α- hydrogen atoms.

