Amines of 12th: It is one of the most important chapter of organic chemistry. Amines are derived by replacing one or more hydrogen atoms of ammonia molecules by alkyl or aryl groups. In nature, amines are present in proteins, vitamins, hormones and nucleic acids. Amines also play a vital role in our daily needs. This chapter is also essential for the students who are preparing for 12th board exams. Every year two or three questions are always asked in the entrance exams like neet, Jee(mains) and IIT advance. We have done our best to include all the important topics of this chapter so I hope that you must read this notes fully.
Amines of 12th: Short and Easy Notes for Amines
What are Amines?
Amines can be considered as the derivatives of ammonia. These organic compounds are obtained by replacement of one, two or all the three hydrogen atoms by alkyl and/ or aryl groups. For example CH3NH2, C6H5NH2, C2H5 – NH – CH3, CH3 – N-(CH3)2.
Structure of Amines
Like ammonia, amine has trivalent nitrogen atom carrying one lone pair of electrons. Nitrogen atom in amine is sp2 hybridised orbitals and its geometry is pyramidal. Due to the presence of unshared electrons in the fourth unhybridised orbitals, the bond angle C – N – E (where E is C or H) is less than 109.5.
Classification of Amines
Depending upon the number of hydrogen atoms replaced by alkyl or aryl groups in ammonia molecules. There are three types of amines
1.Primary amine(1o). If one hydrogen atom of ammonia molecule is replaced by alkyl or aryl group, it is called primary amine. Its general formula is RNH2 where R is alkyl or aryl group.
2. Secondary amine(2o). If two hydrogen atoms of ammonia molecule is replaced by alkyl and/ or aryl group, it is called secondary amine. Its general formula is R2NH where R is alkyl or aryl group.
3. Tertiary amine(3o). If three hydrogen atoms of ammonia molecule is replaced by alkyl and/ or aryl group, it is called tertiary amine. Its general formula is R3N where R is alkyl or aryl group.
Nomenclature of amines
In common system, an aliphatic amine is named alkyl group to amine as alkyl amine. In secondary and tertiary amine, when two or more groups are same. The prefix di or tri is placed before the alkyl group.
In IUPAC system, amines are named as alkanamine. For example CH3NH2 is named as methanamine in case, more than one amine groups are present at different positions in the parent chain. Their positions are specified by giving numbers to the carbon atoms bearing -NH2 groups and suitable prefix as di, tri etc. is attached to the amine. For example H2N – CH2 – CH2 – NH2 is named as ethane – 1,2 – diamine. In aryl amines, the simple compound is C6H5NH2. It is named as Aniline in the common system and benzenamine in IUPAC system.
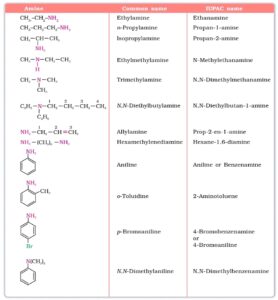
Some examples of nomenclature are given below:—
Preparation of Amines
1. By reduction of nitro group.
Nitro groups are reduced to amines by passing hydrogen gas in the presence of finely divided Ni, Pt or Pd. Nitroalkane can be also reduced to amine by using Sn + HCl or Fe + HCl.
2. By ammonolysis of alkyl halides
An alkyl or benzyl halide on reaction with an ethanolc solution of ammonia undergoes nucleophilic substitution reaction in which the halogen atom is replaced by an amino group to form amine and this process is called ammonolysis.
3. By reduction of nitriles
Nitriles on reduction with LiAlH4 or catalytic hydrogenation produce primary amine.
4. By reduction of amides
The amides on reduction with lithium aluminium hydride give amines.
5. Gabriel phthalimide synthesis
Phthalimide on reaction with ethanolic KOH forms Potassium salt of phthalimide which on heating with alkyl halide followed by alkaline hydrolysis produces the corresponding primary amine. Aromatic primary amine cannot be prepared by this method.
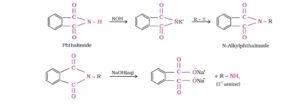
6. Hoffmann bromamide degradation reaction
When an amide is treated with bromine in aqueous or ethanolic solution of Sodium hydroxide, primary amine is obtained. The amine so formed has one carbon atom less than that present in the amide. This process is called Hoffmann bromamide degradation reaction .
Properties of Amines
1. The lower aliphatic amines are gases with fishy odour.
2. Primary amines with three or more carbon atoms are liquids and still higher ones are solid.
3. Aniline and other arylamines are usually colourless but get coloured on storage due to atmospheric oxidation.
4. Lower aliphatic amines are soluble in water but their solubility decreases with increase in molecular masses.
5. The order of boiling point in aliphatic amines are as primary > secondary > tertiary
6. Amines are basic in nature and reacts with acid to form salt.
7. Amines undergo alkylation and acylation to form various types of amines and amides.
8. Aliphatic and aromatic amines on heating with Chloroform and ethanoic Potassium hydroxide form isocynide or carbylamines foul smelling substances. This reaction is called carbylamine reaction.
9. Primary aliphatic amines react with nitrous acid to form alcohols and liberate nitrogen gas but secondary and tertiary amine react in different manners.
10. Aniline reacts with nitrous acid at low temperature to form benzene diazonium salt that is a very important class of compound used for the synthesis of variety of aromatic compounds.
11. Amines react with benzene sulphonyl chlorides known as Hinesburg reagent in different manners hence this reagent is used to distinguish between primary, secondary and tertiary amines.
12. Aniline undergoes electrophilic substitution reactions as bromination, nitration and sulphonation. Aniline does not undergo Friedel crafts reactions because it is already a Lewis base.
Basicity in Amines
1. Amines are Lewis bases and its basic strengths are as following: NH3 < primary < secondary < tertiary.
2. In aqueous solution, basic strength of amines depends on the following factors:
- Inductive effect
- Solvation effect
- Steric hindrance
After consideration all above the facts, the basic strength of amines can be represented as following:
(C2H5)2NH > (C2H5)3N > C2H5NH2 > NH3.
(CH3)2NH > CH3NH2 > (CH3)3NH > NH3
3. It is observed that in case of substituted Aniline, the basic strength of these compounds increases by electrons releasing groups like – OCH3, -CH3 and decreases by electrons withdrawing groups like – NO2, – SO3H, -COOH.
Summary of Amines for 12th
We have learnt in this chapter Amines for 12th that amines are the derivatives of ammonia molecules. There are three types of amines. We also learn that how we can their name either by common method or by IUPAC system. We have also discussed well about the preparation, properties and base nature of amines properly. We hope that this notes will be beneficial for you all. If you have any doubts or suggestions regarding this chapter, you must share with us. If you like this notes please share among your friends and favourites.
FAQ in Amines of 12th
Q.No 1. What amine salts are used for determining their molecular masses?
Ans:- Chloroplatinates
Q.No 2. What is diazotisation ?
Ans:- The process of conversion of atomic primary amine by treatment with NaNO2/HCl at 273 – 278K to arenediazonium salts is called diazotisation.
Q.No 3. What is ammonolysis ?
The reaction of alkyl halides with ammonia or amines to form a mixture of 1o, 2o, 3o amine and quaternary salts is called ammonolysis.
Q.No 4. Why amines are basic in nature?
Dues to the presence of a lone pair of electrons on the N- atom, amines can easily donate the lone pair to an electron deficient species and hence act as a Lewis base.

