Analysis of Organic Compounds is also known as the part of Analytical Chemistry. We shall learn in this notes about detection of various elements present in an organic compound. We shall also find out the percentage of each element present in an organic compound by different methods. This topic is related to the organic chemistry- some basic Principles and Techniques. This is the most important topic of organic chemistry for the students who are preparing for the entrance exams of NEET, Jee(mains),IIT advance. We have discussed this topic in simple and proper ways. Therefore you all must read till end. Let’s start this notes.
Analysis of Organic Compounds
We shall analyse the organic compounds in two ways as qualitative analysis and quantitative analysis. Both the ways will be explained separately.
Qualitative analysis in Analysis of Organic Compounds
This analysis involves the elemental analysis in the organic compounds. As we know that organic compounds consist of mainly carbon and hydrogen but besides them nitrogen, phosphorus, oxygen, halogens and sulphur are also present. We shall detect all of them by the following methods.
1. Detection of carbon and hydrogen. When the organic compound is heated with dry cupric oxide, carbon and hydrogen present in the compound gets oxidised to CO2 and H2O respectively. CO2 turns lime water milky and H2O turns anhydrous copper sulphate blue.
2. Detection of Nitrogen.
Nitrogen is detected by Lassaigne’s Test. In this method, organic compound is fused with sodium metal in a fusion tube. Nitrogen and carbon of the compound react with sodium to form NaCN. Now the red hot tube is broken in distilled water, boiled and filtered, the filtered is called Lassaigne’s extract.
Na + C + N → NaCN
Now this extract is reacted with ferrous sulfate solution, sodium ferrocynide Na4[Fe(CN)6] is obtained as complex compound. On boiling, some of the Fe2+ ions are oxidised to Fe3+ ions by the atmospheric oxygen. On oxidation with dil. H2SO4/dil. HCl appearance of blue colour confirms the presence of nitrogen in the given organic compounds.
4Fe3+ + 3Na4[Fe(CN)6] → Fe4[Fe(CN)6]3 + 12 Na ↑ Ferric ferrocynide (prussian blue)
3. Detection of Sulphur. When sulphur is present, organic compounds change to sodium sulphide as Lassaigne’s extract.
2Na + S → Na2S
Now this extract is reacted in two ways:
With sodium nitroprusside, sodium sulphide gives a violet colouration due to the formation of sodium thionitroprusside
Na2S + Na2[Fe(NO)(CN)5] → Na2[Fe(CN)5NOS]
With lead acetate solution, sodium sulphide reacts to give a black ppt of PbS.
Na2S + (CH3COO)2Pb → PbS + 2CH3COONa.
If both N and S are present, it may result in the formation of NaCNS during fusion with Sodium metal. In acidic medium, NaCNS reacts with Fe3+ ions to give blood red Fe(CNS)3 named as ferric sulphocynide.
3NaCNS + Fe3+ → Fe(CNS)3 + 3Na+
Detection of Halogens. The detection of halogens is done by Lassaigne’s test. In this method first upon all, organic compound is fused with sodium. Now the halogens present in the organic compounds are converted into the corresponding halides of sodium. Later a portion of sodium fusion extract is treated with 1 ml. of conc. HNO3, boiled to decompose sodium cyanide or sodium sulphide present in the extract of the compound contains nitrogen and sulphur.
Finally the solution is cooled and a small amount of AgNO3 is added. The following results indicate the presence of halogens.
- A white ppt. (AgCl) soluble in ammonium hydroxide indicates chlorine.
- A white ppt. (AgBr) sparingly soluble in ammonium hydroxide indicates bromine.
- A white ppt. (AgI) insoluble in ammonium hydroxide indicates Iodine.
Detection of Phosphorous
The organic compound is fused with Sodium peroxide and the mass is extracted with water. The extract is boiled with conc. HNO3 and then ammonium molybdate is added. Formation of a yellow ppt. of ammonium phosphomolybdate (NH4)3PO4.12MoO3 confirms the presence of phosphorus in the given organic compounds.
Read more: 11th Organic Chemistry: Notes Some Basic Principles and Techniques
Quantitative Analysis of Organic Compounds
This analysis involves the estimation of percentage composition of various elements present in an organic compound. We shall now discuss the principles involved in the estimation of various elements usually present in an organic compounds.
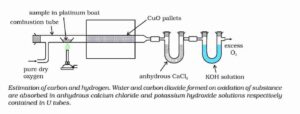
Estimation of carbon and hydrogen
A known mass of organic compound is heated strongly with excess of dry copper oxide in a current of dry air or oxygen. Under these conditions, carbon present in the organic compound is oxidised to carbon dioxide and hydrogen is oxidised to water.
Formula for estimation.
Percentage of carbon = 12×mass of CO2 evolved ×100/44 × mass of organic compound taken.
Percentage of Hydrogen = 2 × mass of H2O formed × 100/ 18 × mass of organic compound taken
Estimation of nitrogen.
The estimation of nitrogen is done by two methods present in an organic compounds. They are Duma’s method and Kjeldahi’s method.
1. Duma’s method. In this method, a known mass of the organic substance is heated with excess of Copper oxide in an atmosphere of CO2. Carbon, hydrogen and sulphur are oxidised to CO2, H2O and SO2 while nitrogen gas is set free. Any oxide of nitrogen that may be formed is reduced back to free nitrogen by passing over a hot reduced copper gauze.
Formula for estimation.
Percentage of Nitrogen = 28 × volume of N2 at STP × 100/ 22400× mass of organic compound taken.
Or, Percentage of Nitrogen = mass of N2 at STP × 100/ mass of organic compound taken.
2. Kjeldahi’s method. In this method a known mass of the organic compound is heated with conc. H2SO4 in presence of Potassium sulphate and a little Copper sulphate or mercury in a long necked flask called Kjeldahi’s flask. As a result of the reaction, the nitrogen present in the organic compound is quantitatively converted into ammonium sulphate. Now this sulphate is boiled with excess of NaOH solution to liberate ammonia gas which is then absorbed in a known excess of standard acid such as H2SO4 or HCl. Finally from the volume of the acid used up and hence the percentage of nitrogen in the organic compound can be calculated.
Formula for estimation.
Percentage of Nitrogen = 1.4 × normality of acid × volume of acid used/ mass of the organic compound taken.
Estimation of halogens.
In this process, a known mass of the organic compound is heated with fuming Nitric acid and a few crystals of silver nitrate in a sealed hard glass tube called carius tube in a furnace. Under this condition, carbon and hydrogen are oxidised to carbon dioxide and water respectively while halogen is converted into silver Silver halide. The precipitate of Silver halide are filtered, washed, dried and weighed. Knowing the mass of the precipitate formed, the percentage of halogen is calculated as follows.
Percentage of halogen in the organic compound = atomic mass of X × mass of AgX × 100/ molar mass of AgX × mass of organic compound taken.
Estimation of Sulphur.
In this process, a known mass of the organic compound is heated with sodium peroxide or fuming nitric acid in a sealed tube (carius tube). Carbon and hydrogen are oxidised to CO2 and H2O while sulphur is oxidised to sulphuric acid which is then precipitated as barium sulphate by adding excess of barium chloride solution. By calculating the mass of barium sulphate we determine the percentage of sulphur in given organic compound.
Percentage of sulphur in an organic compound = 32 × mass of BaSO4 formed × 100/ 233 × mass of organic compound taken.
Estimation of Phosphorous
In this process, a known mass of the organic compound is heated with fuming Nitric acid in a sealed carius tube. Under these condition
Carbon and hydrogen are oxidised to CO2 and H2O respectively. While Phosphorous present in the organic compound is oxidised to phosphoric acid which is precipitated as ammonium phosphomolybdate by heating it with conc. HNO3 and then adding ammonium molybdate. Now this precipitate is filtered, washed, dried and weighed to find the percentage of Phosphorous in the given organic compound.
Percentage of Phosphorous in the given organic compound = 31 × mass of amm. Phosphomolybdate × 100 / 1877 × mass of organic compound taken.
Alternatively, phosphoric acid is precipitated as magnesium ammonium phosphate by adding magnesia mixture. Now this precipitate is filtered, washed, dried and then ignited. The Magnesium pyro phosphate thus formed is weighed and calculate the percentage of Phosphorous in the given organic compound.
Percentage of Phosphorous in organic compound = 62 × mass of Mg2P2O7 formed × 100 / 222 × mass of the organic compound taken.
Estimation of Oxygen
The percentage of oxygen in an organic compound is generally calculated by the difference method. For this, the percentage of all other elements present in the organic compound are added then the subtracted from 100.
Percentage of oxygen in the given organic compound = 100 – (sum of the percentage of all other elements)
Summary of Analysis of Organic Compounds
We hope that you must have read this notes Analysis of Organic Compounds till end. In this notes we have included all the types of analysis weather it is quantitative or qualitative. In both the types of analysis we have learn to detect and estimate the percentage of elements present in an organic compounds. These elements are carbon, hydrogen, oxygen, sulphur, nitrogen and phosphorus. We expect that you all have much liked this notes. Therefore, you all are requested to share this notes among your friends and favourites.
FAQ in Analysis of Organic Compounds
Q.No 1. What is quantitative estimation of elements in organic compounds?
Ans:- Determination of percentage of each element present in an organic compound after a chemical reaction is called quantitative estimation of elements in organic compounds.
Q.No 2. What is the Liebig’s method?
Ans:- This is the method to estimate carbon and hydrogen present in an organic compound by percentage after reaction with oxygen.
Q.No 3. What is Lassaigne’s extract?
Ans:- A small piece of freshly cut sodium is heated gently in a fusion tube till it forms a shinning globule. Now a small amount of the organic compound is added and heated strongly. Later the hot tube is plunged into a china dish having distilled water. Now this China dish is boiled, cooled and then filtered. This filtrate is called Lassaigne’s extract.
Q.No 4. What is the Carius method used for?
Ans:- This method is used to detect the presence of halogens in an organic compounds. In this method, a known mass of the organic substance is heated with fuming Nitric acid and a few crystals of Silver nitrate in a sealed hard glass tube.
Q.No 5. What is the Duma’s method used for?
Ans:- This method is used to find the percentage of nitrogen in an organic compound in analytical chemistry .

