We shall learn Biomolecules Class 12th that will be short and simple notes of NCERT syllabus. As we know that living things grow, sustain and reproduce itself. The important chemical substances need for such like activities are called biomolecules. These biomolecules can be either organic or inorganic compounds. Some important biomolecules are proteins and carbohydrates which are essential constituent of our food. These biomolecules play an important role in life processes. In addition, some other biomolecules like vitamins and mineral salts are also essential for the function of organisms. We shall discuss all the relative topics provided in ncert syllabus regarding biomolecules.
This notes has been written in proper way so that students can be able to give answer of any questions. These questions may be either subjective or objective. This notes has been written in short and simple language so that any student can understand very easily. Biomolecules Class 12th notes is very important for those students who are going to participate either in 12 th board exams or entrance exams of Jee(mains), IIT advance or Neet. This notes will be also beneficial for the students who are going to participate in competitive exams like Railways, SSC, NDA, UPSC etc.
Biomolecules Class 12th: Short and Simple Notes of NCERT
In this notes, we shall study about carbohydrates, proteins, amino acids, vitamins, enzymes and nucleic acids in details. Now we start from carbohydrate.
What is carbohydrate?
Carbohydrate is simply considered as hydrates of carbon. Its general formula is Cx(H2O)y. For example Glucose [C6(H2O)6]. But the molecular formula of some carbohydrates do not fit in this general formula of carbohydrate. For example rhamnose C6H12O5. Hence carbohydrates may be defined as “optically active poly hydroxy aldehydes or ketones or the such compounds which produce on hydrolysis such units on hydrolysis are called carbohydrates. Most common examples are glucose, fructose, sucrose etc.
Classification of carbohydrates
On the basis of their behaviour on hydrolysis, there are mainly three kinds of carbohydrates.
1. Monosaccharides. A carbohydrate that cannot be hydrolysed further to give simpler unit of polyhydroxy aldehyde or ketone is called Monosaccharide. In nature about 20 monosaccharides are known.
2. Oligosaccharides. Carbohydrates which can produce 2 to 10 mono saccharides units on hydrolysis are known as oligosaccharides. They are further classified as disaccharides, trisaccharides. tetrasaccharides etc. For example sucrose, maltose and lactose are disaccharides.
3. Polysaccharide. Carbohydrates which produce a large number of Monosaccharide units on hydrolysis are called polysaccharide. Some common examples are starch, cellulose, gums, glycogen etc.
* On the basis of taste, carbohydrates are classified into two types as follows:
1. Sugars. Carbohydrates which are sweet in taste are called sugars. All the monosaccharides and some disaccharides are sugars.
2. Non-sugars. Carbohydrates which are not sweet in taste are called non- sugars. All the polysaccharides are non- sugars.
* Based on reducing behaviour, carbohydrates have been divided into two categories. They are –
1. Reducing sugars. All those carbohydrates which reduce Fehling solution and Tollens reagents are called reducing sugars. For example glucose, fructose etc.
2. Non- reducing sugars. Those carbohydrates which don’t reduce Fehling solution and Tollens reagents are called Non- reducing sugars. For example polysaccharides and some oligosaccharides.
Classification of Monosaccharides:
On the basis of functional groups, there are two types of Monosaccharides as aldose and ketoes.
Aldose. The Monosaccharides which contain aldehyde group are called aldose and further on the basis of number of carbon atoms they are referred as aldotriose, aldotetrose, aldopentose etc.
Ketose. The Monosaccharides which contain ketone group are called ketose and further on the basis of number of carbon atoms they are referred as ketotriose, ketotetrose, ketopentose etc.
Preparation of Glucose.
1. From sucrose. When sucrose is boiled with dilute HCl or H2SO4 in alcoholic solution, glucose and fructose are obtained in equal ratio.
2. From starch. Glucose is obtained by hydrolysis of starch by boiling with dilute H2SO4 at 393K under pressure. It is a commercial method to obtain glucose.
Structure of Glucose
Glucose is an aldohexose and also known as dextrose. The structure of glucose was assigned on the basis of following evidence:
1. Its molecular formula was found C6H12O6.
2. On prolonged heating with HI, it forms n- hexane. This reaction justifies that all the six carbon atoms in glucose are linked in a straight chain.
3. Glucose reacts with hydroxyl amine to form an oxime and with HCN form cynohydrin. These reactions justify that glucose has a carbonyl group (>C=O).
4. Glucose gets oxidised to gluconic acids on reaction with a mild oxidising agent like bromine water. This reaction indicates that the carbonyl group is present as aldehyde group in glucose.
5. Acetylation of glucose with acetic anhydride gives glucose pentaacetate which confirms the presence of five – OH group in glucose.
6. On oxidation with nitric acid, glucose as well as gluconic acid yield saccharic acid. This indicates a primary alcoholic group in glucose.
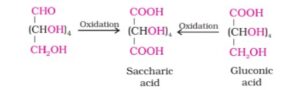
The exact spatial arrangement of different -OH groups was given by
Fischer after studying many other properties. That can be represented in the following way:
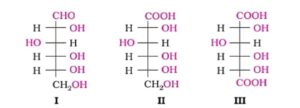
Nomenclature of Glucose. Glucose is correctly named as D(+) glucose. ‘D’ before the name of glucose represents the relative configuration that is similar to the D- form of glyceraldehyde whereas negative (-) sign indicates that glucose is dextrorotatory in optical nature.
Limitations of open chain structure of Glucose.
Open chain structure of glucose explains most of the properties of glucose but the following facts and properties were not explained by this structure.
1. Glucose does not give schiff’s test and it does not form addition product as hydrogen sulphite with NaHSO3 despite having -CHO group.
2. The pentaacetate of glucose does not reacts with hydroxyl amine . This behaviour indicates the absence of free – CHO group in glucose.
3. Glucose is found to exist in two different Crystalline forms as α and β. The α- form of glucose (m.p.419K) obtained by crystallisation from concentrated solution of glucose at 303K. The β- form of glucose(m.p.423K) is obtained by crystallisation from hot and saturated aqueous solution at 371K.
Cylic structure of Glucose
Observing limitations in the open chain structure of Glucose, it was considered that one of the -OH group of glucose may add to the -CHO group and form a cyclic hemiacetal structure. It was found that glucose forms six membered ring in which -OH at C-5 is involved in ring formation. This explains the absence of -CHO group and also existence of glucose in two forms as a- and B- forms.
These two cylic forms exists in equilibrium with open chain structure. The cyclic structure of Glucose is represented by Haworth and resembles with pyran hence its structure is called pyranose structure. These structures can be represented in the following way:
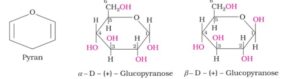
Note- The two cyclic hemiacetal forms of glucose differ only in the Configuration of the hydroxyl group at C1 position. This carbon is called anomeric carbon and these forms of glucose are known as anomers.
Structure of Fructose:
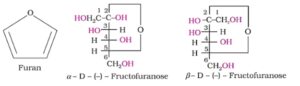
Fructose also has the molecular formula C6H12O6. On the basis of reaction, it was found that it has ketonic functional group at C2 and six carbons in straight chain like glucose. It belongs to D- series and is a laevorotatry compound. It is named as D(-) fructose. It also exists in two cyclic forms by the addition of -OH at C5 to the ketone group present at C2. Its cyclic structure has five membered ring like furan and is named as furanose.
Disaccharides. In a disaccharide, two monosaccharides are joined together by an oxide linkage formed by the loss of water molecules. Such a type of linkage between two monosaccharide units is called glycosidic linkage. The important disaccharides are followings:
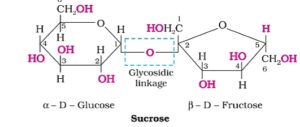
1. Sucrose. In this disaccharide, C1 of a-D- glucose and C2 of B-D- fructose are joined together by glycosidic linkage. Sucrose is a non- reducing sugar. Sucrose is dextrorotatory but after hydrolysis the product is named as invert sugar.
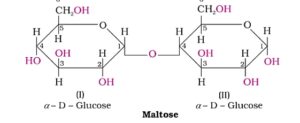
2. Maltose. It is composed of two a- D- glucose units in which C1 of one glucose unit is linked to C4 of another glucose unit. It is a reducing sugar because the free aldehyde group is present at C1 of second glucose unit in solution.
3. Lactose. It is composed of β- D- glactose and β- D- glucose. The linkage is between C1 of galactose and C4 of glucose. It is also a reducing sugar.
Polysaccharides. They contain a large number of monosaccharide units joined together by glycosidic linkage. Some of them are followings:
1. Starch. It is the main storage polysaccharide of plants. It is a polymer of a-glucose and consists of two components as amylose and amylopectin.
Amylose is a water soluble component and it constitutes about 15-20% of starch. It is a long straight chain of α-D-(+) glucose units held together by C1 – C4 glycosidic linkage.
Amylopectin is insoluble in water and constitute about 80-85% of starch. It is a branched chain polymer of α-D-(+) glucose units in which chain is formed C1 – C4 glycosidic linkage in straight chain and branching occurs by C1-C6 glycosidic linkage.
2. Cellulose. It is the most abundant organic substance in plant kingdom. It has straight chain composed of β- D- glucose units which are joined together by glycosidic linkage between C1 of one glucose unit and C4 of the next glucose unit.
3. Glycogen. This carbohydrate is stored in animal body as glycogen. It is also known as animal starch because its structure is similar to amylopectin. It is rather more highly branched. When the body needs glucose. Enzymes break the glycogen down to glucose.
Proteins and their Structure
Proteins are the most abundant biomolecules of the living system. They are found in milk, fish, cheese, pulses, peanuts, meat etc. The word protein is derived from the Greek word, “proteios” which means primary or of prime importance. All proteins are polymers of α- amino acids.
Amino acids. Amino acids contain amino (-NH2) and Carboxyl(-COOH) functional groups. Depending upon the relative position of amino group with respect to the carboxyl group, the amino acids can be α, β, γ, δ and so on. But only a- amino acids are obtained after hydrolysis of proteins.
Classification of Amino acids
1. Based on the nature, amino acids have been classified into three categories.
Acidic amino acids. When carboxyl groups are more than amino groups in an amino acid. It is called acidic amino acid. For example glutanic acid, aspartic acid.
Basic amino acids. When carboxyl groups are Lower than amino groups in an amino acid. It is called basic amino acid. For example lysine, arginine etc.
Neutral amino acids. When carboxyl groups and amino groups are equal in an amino acid. It is called neutral amino acid. For example glycine, alanine etc.
2. Based on presence in living systems, there are two types of amino acids, they are followings:
Essential amino acids. Those amino acids which can not be synthesized in living body are called essential amino acids. For example valine, leucine.
Non essential amino acids. Those amino acids which can be synthesized in living body are called non essential amino acids. For example glycine, alanine.
Structure of Proteins
Proteins are the polymers of α- amino acids and these acids are joined together by peptide bonds or linkage in a large number. Peptide linkage formed when two molecules of similar or different amino acids combine together by losing a molecule of water. The product of this reaction is called dipeptide because it is made of two molecules of amino acids. If a third amino acid combines to a dipeptide, the product is called tripeptide. Protein is a polypeptide in which more than 100 amino acids are joined together by peptide linkage.
Classification of proteins
There are two types of proteins. They are followings:
1. Fibrous proteins. In this protein the polypeptide chains run parallel and are held together by hydrogen or disulphide bonds, then fibre like structure is formed. This type of protein is insoluble in water. For example keratin and myosin.
2. Globular proteins. In this protein the polypeptide chains coil around to give a spherical shape. They are usually soluble in water. For example Insulin and albumin.
Structure and shape of proteins
Structure and shape of proteins can be studied at four levels in the following ways:
1. Primary structure of proteins In this level, each polypeptide in a protein has amino acids linked with each other in a specific sequence.
2. Secondary structure of proteins
This level of structure refers to the shape in which a long polypeptide chain can exist. They are found to exist in two different types of structures as α- helix and β- pleated sheets.
α- helix is one of the most common ways in which a polypeptide chain forms all possible hydrogen bonds by twisting into a right handed screw or helix with the -NH group of each amino acid residue hydrogen bonded to the > CO= of an adjacent turn of helix.
β- pleated sheets. In this structure, all peptide chains are stretched out to nearly maximum extension and then laid side by side which are held together by Intermolecular hydrogen bonds. This structure resembles the pleated folds of drapery and therefore known as β- pleated sheets.
3. Tertiary structure of proteins This structure of proteins represents overall folding of the polypeptide chains. It gives two major molecular shapes like fibrous and globular.
4. Quaternary structure of proteins
Some proteins are composed of two or more polypeptide chains are referred as subunits. The spatial arrangement of these subunits with respect to each other is known as quaternary structure.
Denaturation of proteins: proteins found in a biological system with a unique three dimensional structure and biological activity is called a native protein. When a natural protein is subjected to physical change like change in temperature or chemical change like change in pH, the hydrogen bonds are disturbed and proteins lose its biological activity. This is called denaturation of proteins. For example curdling of milk, coagulation of egg on boiling.
Enzymes
The biological catalysts which increase the chemical process like digestion of foods, absorption of appropriate molecules and ultimately production of energy in a living system are called enzymes. Chemically, all enzymes are globular proteins with high molar mass and form colloidal solution in water. For example invertase, zymase, diastase etc.
Mechanism of enzyme action.
The most accepted mechanism of enzyme catalysed reaction is known as Lock and Key mechanism
Enzymes are highly specific in their action due to the presence of some specific regions, called the active sites on their surface. These active sites are associated with some functional groups such as -NH2, -COOH, -OH, -SH etc. Which form weak bonds such as H- bonds, vander waals attraction etc. with the particular substrate. Thus, the enzyme catalysed reactions take place in two steps as follows:
Step 1. Formation of enzyme- substrate complex.
E + S → ES where E = enzyme, S = substrate and ES = enzyme- substrate complex.
Step 2. Dissociation of enzyme- complex to form the product.
ES → [EP] → E + P, where EP = enzyme-product complex and P = product.
Vitamins
Organic compounds required in the diet in small amounts to perform specific biological functions for normal maintenance of optimum growth and health of the organism are called vitamins. They are Vitamin A, B, C etc.
Classification of vitamins: There are two types of vitamins based on their solubility in water.
1. Fat soluble vitamins. Vitamins which are soluble in fats and oils but insoluble in water are called Fat soluble vitamins. For example Vitamin A, D, E and K.
2. Water soluble vitamins. Vitamins which are soluble in water are called water soluble vitamins. They are Vitamin B and vitamin C except Vitamins B12.
Nucleic Acids
The particles in the nucleus of a cell, responsible for heredity are called chromosomes which are made up of proteins and another type of biomolecules called nucleic acids. These are mainly of two types.
1. Deoxyribonucleic acid (DNA)
2. Ribonucleic acid (RNA)
Chemical composition of nucleic acids:
complete hydrolysis of nucleic acids yields a pentose sugar, phosphoric acid and nitrogen containing heterocyclic compounds called bases. In DNA molecules, the sugar moiety is β- D-2- hydroxy ribose whereas in RNA, it is β- D- ribose. DNA contains four bases adenine (A), guanine (G), cytosine (C) and Thymine (T). RNA also contains four bases, the first three bases are same as in DNA but the fourth one is uracil (U).
Structure of nucleic acids
A structure is formed by the linkage of a base to 1′ position of sugar is known as nucleoside. When nucleoside is linked to phosphoric acid at 5′ position of sugar moiety, it is called nucleotide. These nucleotides joined together in a large number by phosphodiester linkage and form primary structure of nucleic acids.
James Watson and Francis Crick gave a double strand helix structure for DNA. Two nucleic acid chains are wound about each other and held together by hydrogen bonds between pair of bases. The two strands are complimentary to each other. Adenine forms hydrogen bonds with thymine and cytosine forms hydrogen bond with guanine.
In secondary structure of RNA, single stranded helix is present which sometimes foldback on itself RNA molecules are of three types and they perform different functions. They are named as messenger RNA (m- RNA), ribosomal RNA (r- RNA) and transfer RNA (t- RNA).
Summary of Biomolecules Class 12th
In this notes
We have discussed all the topics prescribed by ncert books. We explained about carbohydrates, proteins, amino acids, enzymes, vitamins and nucleic acids. These compounds are collectively known as biomolecules. We have discussed in details about their classification and proper structure. During writing this notes, the proper care was given towards the correctness and simplicity of language so that any reader can easily understand the notes and take maximum benefits in a short time.
We hope that you must have learnt and liked this notes after complete reading. Hence we shall request you all to share this notes among your friends and favourites.
FAQ in Biomolecules Class 12th
Q.No 1. What is invert sugar?
Ans:- The equimolar mixture of glucose and fructose is called invert sugar.
Q.No 2. What is mutarotation?
Ans:- The spontaneous change of specific rotation of an optically active substances with time is called mutarotation.
Q.No 3. What are zwitter ions?
Ans:- A zwitter ion is a dipolar ion formed by neutralization of acidic and basic centres present within a molecule.
Q.No 4. What is isoelectric point?
Ans:- The pH at which there is no net migration of the amino acid under the influence of an applied electric field is called isoelectric point.
Q.No 5. Name the purines present in DNA.
Ans:- adenine and guanine.

