Chemical Bonding Class 11 NCERT Solution: Here we shall read about the correct answer of all the questions being provided in the ncert books of chemistry for class 11th. There are 40 questions in the exercise of this chapter. All the questions are important and include all the concepts and theories which are important to describe the structure of molecules and ions. Answers of all the questions have been solved in simple manners and easy methods so that any student can enhance their knowledge in this chapter very easily.
Chemical Bonding Class 11 NCERT Solution: Free PDF Download for 2025
Ncert solution of any chapter is very helpful for any student. This solution guides students that how much command they have in the various topics of any chapter. Exercise questions give a litmus test of students about preparation for this chapter. Chemical bonding is considered as the basic chapter of chemistry. This chapter makes interesting in understanding the whole chemistry. Here, we learn about the formation of the different compounds and their structure with the help of various concepts and theories. Hence we must know the answer of all the questions given in this chapter.
Chemical Bonding Class 11 NCERT Solution start from here
Write answer of the following questions
Q.No 1. Explain the formation of a chemical bond.(Chemical Bonding Class 11 NCERT Solution)
Ans:- The attraction force through which constituent particles are held together in a molecule/ion is called chemical bond. According to Kossel-Lewis approach, atoms combine together in order to complete their respective octets so as to acquire the stable inert gas configuration.
This can occur in two ways: 1. By complete transfer of one or more electrons from one atom to another atom. 2. By mutual sharing of electrons between two or more atoms. Sometimes a pair of electrons are contributed by one atom but shared by both.
Q.No 2. Write Lewis dot symbols for atoms of the following elements: Mg, Na, B, O, N, Br.
Ans:- 
Q.No 3. Write Lewis symbols for the following atoms and ions:(Chemical Bonding Class 11 NCERT Solution)
S and S2–; Al and Al3+; H and H–
Ans:- 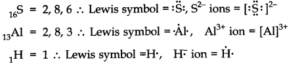
Q.No 4. Draw the Lewis structures for the following molecules and ions:
H2S, SiCl4, BeF2,CO32-, HCOOH
Ans:- 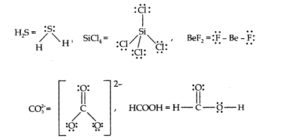
Q.No 5. Define octet rule. Write its significance and limitations.
Ans:- According to Lewis theory, the atoms of different elements combine with each other in order to have 8 electrons in their outermost shell like inert gases. This is called octet rule.
Significance: This octet rule help us to understand why atoms combine together to form chemical bonds. It also suggests that chemical bonds will be Electrovalent or Covalent.
Limitations of octet rule: there are following limitations of octet rule.
- Formation of compounds by hydrogen.
- Formation of compounds like BeCl2, BF3, AlCl3 etc.
- Formation of compounds like PCl5, SF6, IF7, H2SO4 etc.
- Formation of compounds by Noble gases like XeF2, XeF6, XeF4, KrF2 etc.
- Odd electron bonds or molecules like NO, NO2, O2, O2‾.
Q.No 6. Write the favourable factors for the formation of ionic bond.
Ans:- There are three favourable factors for the formation of ionic bonds:
- Ionisation enthalpy. Lower the values of ionisation enthalpies, greater the chances of Ionic bond formation.
- Electron gain enthalpy. Higher is the negative value of electron gain enthalpy, more is the probability of formation of ionic bonds.
- Lattice enthalpy. The higher the value of lattice enthalpy of the resulting ionic compound, the greater is the stability of the compound and hence greater will be the ease of its formation.
Q.No 7. Discuss the shape of the following molecules using the VSEPR model:(Chemical Bonding Class 11 NCERT Solution)
BeCl2, BCl3, SiCl4, AsF5, H2S, PH3
Ans:- 
Q.No 8. Although geometries of NH3 and H2O molecules are distorted tetrahedral, bond angle in water is less than that of ammonia. Discuss.
Ans:- In NH3, N atom has one lone pair of electrons . In H2O, O atom has two lone pair of electrons. The greater is the number of lone pair of electrons around the central atom, the lesser is the bond angle hence bond angle in water is less than that of ammonia. 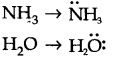
Q.No 9. How do you express the bond strength in terms of bond order?
Ans:- Bond order represents the number of bonds between two atoms. The number of bonds between two atoms increases their bond dissociation enthalpy increases hence bond strength also increases.
Q.No10. Define the bond length.(Chemical Bonding Class 11 NCERT Solution)
Ans:- The equilibrium distance between the centres of the nuclei of the two covalently bonded atoms is called its bond length. It is expressed in terms of Ao or pm. For example bond length in H — H = 74 pm.
Q.No11. Explain the important aspects of resonance with reference to the CO32– ion.
Ans:- 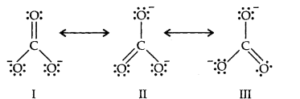
All these three structure of C032– are resonating structures because
- The position of atoms are same.
- Number of unshared electrons are same but they differ in their position.
- The resonance energy of all the structures are same because they have equal number of shared pair of electrons.
Q.No 12. H3PO3 can be represented by structures 1 and 2 shown below. Can these two structures be taken as the canonical forms of the resonance hybrid representing H3PO3? If not, give reasons for the same.
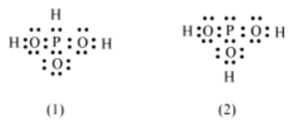
Ans:- These two structures of H3PO3 cannot be canonical of resonance hybrid because position of H atom is different which violates the concept of resonance.
Q.No 13. Write the resonance structures for SO3, NO2 and NO3–. (Chemical Bonding Class 11 NCERT Solution)
Ans:- 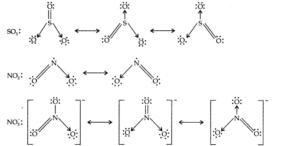
Q.No 14. Use Lewis symbols to show electron transfer between the following atoms to form cations and anions: (a) K and S (b) Ca and O (c) Al and N.
Ans:- 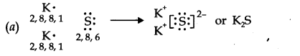
Q.No 15. Although both CO2 and H2O are triatomic molecules, the shape of H2O molecule is bent while that of CO2 is linear. Explain this on the basis of dipole moment. (Chemical Bonding Class 11 NCERT Solution)
Ans:- CO2 is linear because its dipole moment is zero. In this molecule, two C=O are present at opposite directions which cancel out the dipole moment of each polar bond. H2O is bent because its dipole moment is 1.84 D. In this molecule, O – H bonds are directed at 104.5o hence not cancel out the dipole moment of each bond and hence its structure is bent.
Q.No 16. Write the significance / applications of dipole moment.
Ans:- Followings are the significance or applications of dipole moment
- In determining the polarity of bonds. When dipole moment of a molecule is zero, it is non polar and when greater than zero the molecule is polar.
- In the calculation of percentage Ionic character.
- In determining the symmetry or shape of the molecule
- To distinguish between cis and trans isomers.
- To distinguish between ortho, meta and para isomers.
Q.No 17. Define electronegativity. How does it differ from electron gain enthalpy? (Chemical Bonding Class 11 NCERT Solution)
Ans:- Electronegativity refers to the tendency of the atom of an element to attract the shared pair of electrons towards it in a covalent bond whereas electron gain enthalpy refers to the tendency of an isolated gaseous atom to accept an additional electron to form a negative ion.
Q.No 18. Explain with the help of suitable example polar covalent bond.
Ans:- When two dissimilar atoms having different electronegativities combine together to form a covalent bond, the shared pair of electrons shifts towards the atom having greater electronegativity. As a result, one end of the molecule, having more electronegative atom becomes slightly negatively charged while the other end acquires slightly positive charge. Thus positive and negative poles are developed and this type of bond is called polar covalent bond. For example 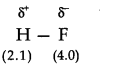
Q.No 19. Arrange the bonds in order of increasing ionic character in the molecules: LiF, K2O, N2, SO2 and ClF3.
Ans:- N2 < SO2 < ClF3 < K2O < LiF.
Q.No 20. The skeletal structure of CH3COOH as shown below is correct, but some of the bonds are shown incorrectly. Write the correct Lewis structure for acetic acid. (Chemical Bonding Class 11 NCERT Solution)
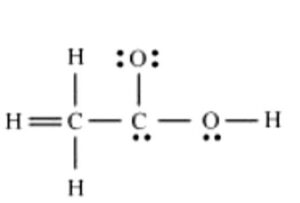
Ans:- 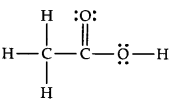
Q.No 21. Apart from tetrahedral geometry, another possible geometry for CH4 is square planar with the four H atoms at the corners of the square and the C atom at its centre. Explain why CH4 is not square planar?
Ans:- The hybridisation of carbon in CH4 is sp3 and bond angle is 109o28′. Therefore, its geometry is tetrahedral. The central atom of CH4 is the element of 2nd period and hence it has not any d- orbitals in valence shell. Therefore, the hybridisation of CH4 cannot be dsp2 to be geometry square planar.
Q.No 22. Explain why BeH2 molecule has a zero dipole moment although the Be–H bonds are polar. (Chemical Bonding Class 11 NCERT Solution)
Ans:- This is because BeH2 molecule is linear (H — Be — H) so that the two Be – H bond moment are equal and opposite and hence cancel out.
Q.No 23. Which out of NH3 and NF3 has higher dipole moment and why?
Ans:- NH3 has higher dipole moment than NF3. The reason is that F is more electronegative than N, therefore direction of bond is from N to F and opposite to the direction of lone pair of electrons in NF3 whereas N is more electronegative than H in NH3, the direction of the bond is from H to N and to the same direction of lone pair of electrons. As a result, the net dipole moment of NH3 is higher than NF3.
Q.No 24. What is meant by hybridisation of atomic orbitals? Describe the shapes of sp, sp2, sp3 hybrid orbitals.
Ans:- The mixing of the atomic orbitals belonging to the same atom but having slightly different energies so that a redistribution of energy takes place between them resulting in the formation of new orbitals of equal energies and identical shapes is called hybridisation. The new orbitals thus formed are called hybrid orbitals.
Sp hybridisation. When one s and one p orbital of the valence shell mix together to form two new equivalent orbitals. These orbitals are called sp hybrid orbitals. The shape of this hybridisation is linear and bond angle is 180o. For example all compounds of beryllium like BeF2, BeH2 etc.
sp2 hybridisation. In this hybridisation one s and two p orbitals of valence shell intermixed together. The shape of this hybridisation is triangular planar and bond angle is 120o. For example all compounds of boron like BF3, BH3 etc.
sp3 hybridisation. In this hybridisation one s and three p orbitals of valence shell intermixed together. The shape of this hybridisation is tetrahedral and bond angle is 109o28. For example CH4, CCl4 etc.
Q.No 25. Describe the change in hybridisation (if any) of the Al atom in the following reaction. (Chemical Bonding Class 11 NCERT Solution)
AlCl3 + Cl– → AlCl–4
Ans:- Electronic configuration of 13Al = 1s2 2s2 2p6 3s1 3px13py1 is in (excited state) in AlCl3. This configuration indicates that one s and two p orbitals are only involved in hybridisation hence the hybridisation of the Al atom is sp2. In AlCl–4 the E.C. of Al in exited state is 1s2 2s2 2p6 3s1 3px13py1 3pz1 . Here one s and three p orbitals are involved in hybridisation hence the hybridisation of Al atom becomes sp3.
Q.No 26. Is there any change in the hybridisation of B and N atoms as a result of the following reaction?
BF3 + NH3 → F3B.NH3
Ans:- Yes, there is change in the hybridisation of B not in N atom before the reaction and after the reaction. In reactant side, the hybridisation of B atom is sp2 and in product side is sp3.
Q.No 27. Draw diagrams showing the formation of a double bond and a triple bond between carbon atoms in C2H4 and C2H2 molecules.
Ans:- 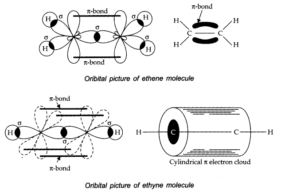
Q.No 28. What is the total number of sigma and pi bonds in the following molecules? (Chemical Bonding Class 11 NCERT Solution)
(a) C2H2 (b) C2H4
Ans:- (a) The structural formula of C2H2 is H– C ≡ C – H. Here the sigma bonds = 3 and pi bonds = 2.
The structural formula of C2H4 is 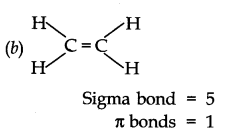
Q.No 29. Considering x-axis as the internuclear axis which out of the following will not form a sigma bond and why? (a) 1s and 1s (b) 1s and 2px (c) 2py and 2py (d) 1s and 2s.
Ans:- only (c) will not form a sigma bond because taking x- axis as the internuclear axis, there will be lateral or sideways overlapping between the two 2py orbitals forming a pi bond.
Q.No 30. Which hybrid orbitals are used by carbon atoms in the following molecules? (Chemical Bonding Class 11 NCERT Solution)
CH3–CH3; (b) CH3–CH=CH2; (c) CH3-CH2-OH; (d) CH3-CHO (e) CH3COOH
Ans:- 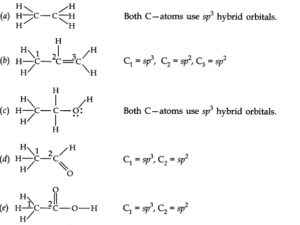
Q.No 31. What do you understand by bond pairs and lone pairs of electrons? Illustrate by giving one example of each type.
Ans:- Those pair of electrons present in the valence shell of any atom are shared between two atoms during formation of covalent bond are called bond pairs of electrons. The pair of valence shell electrons which do not involved in the formation of covalent bonds are called lone pair of electrons. Bond pair of electrons are represented by solid dash and lone pair of electrons by two dots around the bonded atoms. 
Q.No 32. Distinguish between a sigma and a pi bond. (Chemical Bonding Class 11 NCERT Solution)
Ans:- 
Q.No 33. Explain the formation of H2 molecule on the basis of valence bond theory.
Ans:- In case of hydrogen, the magnitude of the attractive forces is more than that of the repulsive forces. As a result, the potential energy of the system decreases and a molecule of hydrogen is formed.
Q.No 34. Write the important conditions required for the linear combination of atomic orbitals to form molecular orbitals. (Chemical Bonding Class 11 NCERT Solution)
Ans:- There are the important conditions for the combination of atomic orbitals to form molecular orbitals.
- The combining atomic orbitals should have comparable energies that means 1s atomic orbital of one atom can combine with 1s atomic orbital of another atom.
- The combining atomic orbitals must have proper orientation so that they are able to overlap to a considerable extent.
- The extent of overlapping should be large. Greater the overlap, greater will be the electron density between the nuclei.
Q.No 35. Use molecular orbital theory to explain why the Be2 molecule does not exist.
Ans:- 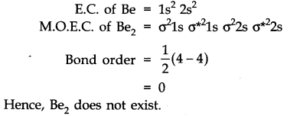
Q.No 36. Compare the relative stability of the following species and indicate their magnetic properties;
O2,O2+,O2– (superoxide), O22- (peroxide)
Ans:- Bond order = (nb – na) /2 hence Bond order in O2 = (10 – 6 ) /2 = 2 and unpaired electrons in the last anti bonding molecular orbitals = 2 hence it is paramagnetic.
Bond order in O2+ = (10 – 5 ) /2 = 2.5 and unpaired electrons in the last anti bonding molecular orbitals = 1 hence it is paramagnetic.
Bond order in O2– = (10 – 7 ) /2 = 1.5 and unpaired electrons in the last anti bonding molecular orbitals = 1 hence it is paramagnetic.
Bond order in O22– = (10 – 8 ) /2 = 1 and unpaired electrons in the last anti bonding molecular orbitals = 0 hence it is diamagnetic.
The stability of these species is directly proportional to the bond order hence the relative order of stability is O2+ > O2 > O2– > O22- .
Q.No 37. Write the significance of a plus and a minus sign shown in representing the orbitals. (Chemical Bonding Class 11 NCERT Solution)
Ans:- As orbitals are represented by wave functions, a plus sign in an orbital represents a +ve wave function and a minus sign represents a –ve wave function. In other words, Plus (+ve) sign denotes crest, while (-ve) sign denotes trough.
Q.No 38. Describe the hybridisation in case of PCl5. Why are the axial bonds longer as compared to equatorial bonds?
Ans:- The electronic Configuration of Phosphorous in ground state and exited state are given below:—- 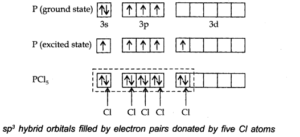
The one s, three-p and one d-orbitals hybridise together to produce five sets of SP3 d hybrid orbitals which are directed towards the five corners of a trigonal bipyramidal as in Fig. 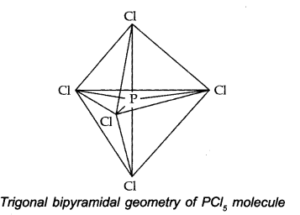
Because axial bond pairs suffer more repulsive interaction from the equatorial bond pairs, therefore axial bonds have been found to be slightly longer and hence slightly weaker than equatorial bonds.
Q.No 39. Define hydrogen bond. Is it weaker or stronger than the van der Waals forces? (Chemical Bonding Class 11 NCERT Solution)
Ans:- Whenever a molecule contains a hydrogen atom linked to a highly electronegative atom like F, O or N, this atom attracts the shared pair of electrons more and so this end of the molecule becomes slightly negative while the other end of hydrogen becomes slightly positive. The negative end of one molecule attracts the positive end of the other and as a result, a weak bond is formed between them. This bond is called hydrogen bond. It is represented by dotted lines.
Hydrogen bond is stronger than vander waals force.
Q.No 40. What is meant by the term bond order? Calculate the bond order of: N2, O2,O2+ and O2–.
Ans:- Bond order. The half of the difference between the number of electrons present in the bonding and the anti bonding orbitals is called bond order.
Bond order = (nb – na) /2 Where nb = electrons of bonding molecular orbitals and na = electrons of anti bonding molecular orbitals
Bond order in N2 = (10 – 4)/2 = 3
Bond order in O2 = (10 – 6 ) /2 = 2
Bond order in O2+ = (10 – 5 ) /2 = 2.5
Bond order in O2– = (10 – 7 ) /2 = 1.5
Conclusion of Chemical Bonding Class 11 NCERT Solution
In this solution notes, we have solved all the questions of chemical bonding. Chemical Bonding Class 11 NCERT Solution will be very helpful to take help during solving the problems of the questions given in the exercise of chemical bonding for class 11th. There are total 40 questions in this exercise. The answer of all the questions have been written in simple manner and proper way.
We hope that this solution notes will be very helpful and useful to you all. We request you all that share this article among your friends and favourites. Thank you for your kind attention.

