Chemical Equilibrium Notes has been written so short and simple that any readers can easily understand this chapter. In this notes all the important topics have been covered. these topics will be very useful for the students who are preparing for NEET, JEE(Mains), IIT and other competitive exams. Equilibrium in physical and chemical processes, Dynamic nature of equilibrium, Laws of mass action, Equilibrium constant, Factors affecting Equilibrium and Le Chatelier Principle are the topics which have been discussed in this notes.
Chemical Equilibrium Notes in details
What is called equilibrium?
The state of a process in which the properties like temperature, pressure, concentration of the system do not show any change with the passage of time is called Equilibrium. To attain equilibrium in any process, the rates of the two opposing processes become equal.
Types of equilibrium
We have to read three types of equilibrium in physical chemistry
1. Physical equilibrium. If the two opposing processes involve physical changes, it is called physical equilibrium.
For example, Solid ⇔ Liquid.
2. Chemical equilibrium. If the two opposing processes involve chemical changes, it is called chemical equilibrium.
For example, PCl5 ⇔ PCl3 + Cl2
3. Ionic equilibrium. If the two opposing process involve partially dissociation of weak electrolyte into ions. it is called ionic equilibrium.
For example, HCl + H2O ⇔ H3O+ + Cl–
Some features of Physical Equilibria
1. Solid ⇌ liquid, H2O (s) ⇌ H2O (l)
in this process, equilibrium exists only at one particular temperature, that is melting or freezing point. Thus, for such an equilibrium, temperature is constant.
2. Liquid ⇌ gas, H2O (l) ⇌ H2O (g)
in this equilibrium, the pressure of the vapours above the liquid is constant at constant temperature.
3. Solid ⇌ solution,
sugar(s) ⇌ sugar (solution)
in this equilibrium, the solubility of the solid in the solution is constant at constant temperature.
4. Gas ⇌ solution, CO2 (g) ⇌ CO2 (aq)
in this equilibrium, the mass of the gas dissolved is constant for constant equilibrium pressure at constant temperature.
General characteristics of Equilibria involving physical processes
1. At equilibrium, some observable property of the system becomes constant.
2. Equilibria involving gases can be attained only in closed vessels.
3. Equilibrium is dynamic in nature.
4. At equilibrium, the concentration of the different substances become constant at constant temperature.
5. At equilibrium, there exists an expression involving the concentration of the substances which is constant at constant temperature and this constant is called equilibrium constant.
6. The magnitude of the equilibrium constant represents the extent to which the process proceeds before equilibrium is attained.
Concept of Chemical Equilibrium
Chemical equilibrium takes place in reversible reactions. This is the reaction in which not only the reactants react to form products under certain conditions but also the products react to form reactants under the same conditions. It is represented by putting a double arrow (⇌) between the reactants and the products. At the chemical equilibrium, the rate of forward reaction becomes equal to the rate of forward reaction. For example A + B ⇌ C + D.
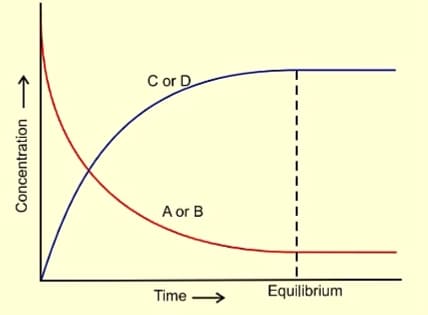
Characteristics of Chemical Equilibrium
- At equilibrium, the concentration of each of the reactants and the products become constant.
- At equilibrium, the rate of forward reaction becomes equal to the backward reaction and hence the equilibrium is dynamic in nature.
- A chemical equilibrium can be established only if none of the products is allowed to escape out or separate out as a solid.
- Chemical equilibrium can be obtained from any direction.
- A catalyst does not change the state of equilibrium.
Law of Mass Action.
The rate of a reaction is proportional to the product of the active masses of the reacting substances, each raised to the power equal to its stoichiometric coefficient as represented by the balanced chemical equation.
Mathematical expression of Law of Mass Action
Consider the reaction
2A + 3B → Products
Rate of reaction α [A]2[B]3 or rate of reaction = k [A]2[B]3
Law of Chemical Equilibrium.
According to this law, the rate of the forward reaction is equal to the rate of the backward reaction in a particular reversible reaction.
For example 2A + 3B ⇌ 4C + 5D
Rf = Kf [A]2[B]3 and Rb = Kb [C]4[D]5
According to Law of Chemical Equilibrium, Rf = Rb
Kf /Kb = [C]4[D]5/[A]2[B]3
∴ Kc = Kf /Kb, and Kc = [C]4[D]5/[A]2[B]3
Where Kc is known as equilibrium constant.
In case of gaseous reactions, when pressure is applied in place of concentration terms, equilibrium constant is represented by Kp and a
KP = P4C.P5D /P2A.P3B
Relation between KP and Kc
KP = Kc . (RT) Δn Where Δn = difference between total no of moles of products and reactants present in gaseous state.
Characteristics of Equilibrium Constant
1. The value of the equilibrium constant for a particular reaction is always constant depending only upon the temperature of the reaction is independent of the concentration of the reactants with which we start or the direction from which the equilibrium is obtained.
2. If the reaction is reversed, the value of the equilibrium constant is inversed. For example
CH3COOH + C2H5OH ⇌ CH3COOC2H5 + H2O, Kc = 4.0
If the above reaction is written in the reverse manner as –
CH3COOC2H5 + H2O ⇌ CH3COOH + C2H5OH, K’c = 1/Kc = 1/4.
3. If the equation having equilibrium constant K, is divided by n, the equilibrium constant for the new reaction is the K1/n.
4. If the equation having equilibrium constant K, is multiplied by n, the equilibrium constant for the new reaction is the Kn.
5.If the equation having equilibrium constant K, is written in two or more steps then K1 × K2 × —- × Kn = K.
6. The value of the equilibrium constant is not affected by the addition of a catalyst to the reaction.
Types of Chemical Equilibria
There are two types of chemical equilibria
1. Homogeneous equilibria:
When in an equilibrium reaction, all the reactants and the products are present in the same phase, it is called a homogeneous equilibrium.
For example:
H2(g) + I2 (g) ⇌ 2HI(g)
N2(g) + 3H2(g) ⇌ 2NH3 (g)
2. Heterogeneous equilibria:
When in an equilibrium reaction, all the reactants and the products are present in two or more than two phases, it is called heterogeneous equilibria.
For example:
CaCO3 (s) ⇌ CaO (s) + CO2(g).
C(s) + H2O (g) ⇌ CO(g) + H2(g)
Application of Equilibrium Constant
1. How to predict the extent of reaction. The magnitude of the equilibrium constant gives an idea of the relative amounts of the reactants and the products.
- Large value of the equilibrium constant (>103) shows that forward reaction is favoured. That means the concentration of products is much larger than that of the reactants at equilibrium.
- Intermediate value of K (10-3 to 103) shows that the concentration of the reactants and the products are comparable.
- Low value of K (<10-3) shows that backward reaction is favoured. That means concentration of reactants is much larger than that of the products.
2. To predict the direction of reaction. For the reaction
aA + bB —- xC + yD
At any stage of the reaction, other than the stage of chemical equilibrium, concentration ratio, as given by the expression for the law of chemical equilibrium is called concentration quotient or reaction quotient. It is usually represented by Qc. Hence Qc = [C]x[D]y/[A]a[B]b
- If Qc = Kc, the reaction is in equilibrium
- If Qc > Kc, the reaction will proceed in the backward direction.
- If Qc < Kc, the reaction will proceed in the forward direction.
3.To calculate equilibrium concentration
By knowing the initial concentration of reactants, equilibrium concentration of all the reactants and the products can be calculated through the following steps.
Step 1. Write the balanced equation for the reaction.
Step 2. Assume x as the amount of the reactants reacted or a product formed.
Step 3. Calculate the equilibrium concentration of each reactant and product from the stoichiometry of the reaction.
Step 4. Write the expression for Kc or Kp.
Step 5. Substitute equilibrium concentrations and calculate x.
Step 6. Check the result by substituting calculated values of equilibrium concentration to get the value of Kc or Kp.
Factors Affecting Equilibrium
The effect of change of pressure, concentration and temperature is predicted with the help of Le Chatelier’s principle. This principle implies that:
If a system in equilibrium is subjected to a change of pressure, concentration or temperature, the equilibrium shift in a direction that tends to undo the effect of the change imposed.
On the basis of Le Chatelier’s principle, factors in a system affect the equilibrium in following ways:
1. Change of concentration of any reactant or product.
If in a reaction present in equilibrium, the concentration of any reactant is increased the equilibrium shifts in the forward direction. On the other hand, if the concentration of any product is increased, the equilibrium shifts in the backward direction. The reverse happens if the concentrations are decreased.
2. Change in temperature.
Exothermic reactions are favoured by low temperature whereas endothermic reactions are favoured by high temperature. That means at low temperature, the rate of exothermic reaction increases while at high temperature, the rate of endothermic reactions increases.
3. Change in pressure.
Low pressure favours those reactions which are proceeds with increase in total number of moles and high pressure favours those reactions which take place with decrease in total number of moles. However, pressure has no effect on an equilibrium reaction which proceeds with no change in total number of moles.
4. Effect of catalyst on the equilibrium:
The addition of a catalyst does not disturb the equilibrium. However, it helps in the attainment of equilibrium quickly.
5. Effect of adding an inert gas to a reaction mixture in equilibrium.
Addition of inert gas at constant Volume has no effect on the state of equilibrium whereas at constant pressure the equilibrium shifts towards large number of moles.
Note. In case of gaseous reactions when np = nr, there is no change in the number of moles, therefore, there is no effect of adding an inert gas on state of equilibrium.

