Coordination compounds is the last chapter of 12th chemistry. In coordination compounds notes for class 12th in new style, we shall study about the formation of coordination compounds, some important terms which will help to understand and identify the basic natures and properties of coordination compounds. We shall also learn about various laws like Warner’s theory, valence bond theory, crystal field theory which will help us to understand the character, structure and properties of coordination compounds very well.
This chapter is very much important for every kind of students who are going to participate in entrance exams for jee (mains), IIT advance, Neet and NDA exams. This chapter is very crucial for the students who are going to sit for the CUET Exams. This chapter is very interesting also because it is factual and basic chapter that is also known as organic chemistry in inorganic chemistry.
coordination compounds notes for class 12th in new style
Coordination compounds are those compounds which have coordinate bonds between central metal atom and ligands. Central metal atom or ion and ligands are enclosed together inside square bracket during representation as formula and this is not Ionisable during dissolution in water hence this part is called complex entities. These entities may be positive, negative or even neutral. For example. [Co(H2O)6]3+ is a positive complex,
[Fe(CN)6]3- is a negative complex and [Ni(CO)4] is a neutral complex.
Some important characteristics of coordination compounds
- These compounds are formed by mixing two or more simple salts in any ratio.
- They exist in the solid state as well as in aqueous solution.
- They may or may not be ionic but the complex part always contains coordinate bonds.
- The properties of the coordination compounds are different from its constituents.
- In a coordination compound, the metal atom or ion is satisfied by two types of valencies called primary valency and secondary valencies
- A coordination compound retains its identity in the solution.
Double salts: The addition compounds which exist in solid state but losses their identity in its aqueous solution are called double salts. For example Mohr’s salt FeSO4.(NH4)2SO4.6H2O, carnallite KCl.MgCl2.6H2O.
Important characteristics of double salts:
- They usually contain two simple salts in equimolar proportions.
- They exist only in solid states and dissociate into ions in its aqueous solution.
- They are mostly ionic compounds and do not contain any coordinate bonds.
- The properties of the double salts are same form its constituents.
- In a double salts, the metal ions show their normal valencies.
- A double salt losses its identity in the solution.
Some important terms in coordination compounds
1. Ligands: The atoms, molecules or anions which donate a pair of electrons to the metal atom or ion and form coordinate bond with it are called ligands. For example H2O, NH3, Cl–, CN–, C2O42- etc.
Ligands can be Unidentate, bidentate, tridentate or polydentate. If only one donor atom is present in a ligand, it is called monodentate. Similarly having two sonar atoms are called bidentate and for three or more are called polydentate. EDTA is hexadentate or polydentate ligand.
Denticity: The number of ligating or coordinating groups or atoms present in a ligand is called its denticity. For example, denticity of CN– =1 and it is a monodentate ligand. Denticity of ethylene diamine (NH2.CH2.CH2.NH2) = 2 and it is di or bidentate ligand. The denticity of EDTA is six.
Chelation: A ligand may contain two donor atoms positioned in such a way that a five or six membered ring is formed with the metal ion, this property is called chelation and the ring is called chelate ring. This ligand is called chelating ligand. For example ethylene diamine is an example of chelating ligand.
Ambidentate ligands: Unidentate ligands containing more than one coordinating atoms are called ambidentate ligands. For example -NO2 and -SCN.
2. Central metal atom or ion: The metal atom linked with ligands by coordinate bonds is called central metal atom or ion.
3. Counter ions: The ionisable ions present in a coordination compounds are called counter ions. They can be cations or anions. They are written outside of square bracket in molecular formula of coordination compounds.
4. Coordination sphere or entity. The central atom and the ligands which are directly attached to it are enclosed in square brackets and are collectively called coordination sphere or entity.
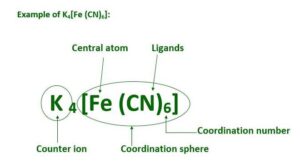
5. Coordination Polyhedron. The spatial arrangement of the ligands which are directly attached to the central atom or ion is called coordination polyhedron. These arrangements can be tetrahedral, square planar, octahedral etc.
6. Oxidation number or state. The oxidation number is the charge assigned on the central metal atom with respect to the total charge on ligands and on the coordination sphere. For example the oxidation number of Fe in [Fe(CN)6]4- is 6 – 4 = +2.
7. Coordination number. It is the number obtained after the sum of the products of no. of ligands × denticity. C.NO. = E no. of ligands × density of this ligand. For example coordination number of Co in [Co(en)2(NH3)2]Cl3 is 2×2 + 2×1 = 8.
8. Homoleptic and Heteroleptic complexes: Complexes in which the metal atom or ion is linked to only one type of ligands are called homoleptic complexes, e.g., [Co(NH3)6]3+. The complexes in which the metal atom or ion is linked to more than one kind of ligands are called heteroleptic complexes, e.g. [Co(NH3)4Cl2]+.
9. Homonuclear and Polynuclear complexes. Complexes in which only central metal atom is present are known as homonuclear complexes. All the examples given so far are homonuclear complexes. Complexes in which more than one metal atoms is present are known as polynuclear complexes.
10. Effective atomic number (EAN): The number of electrons present in the metal ion in a complex plus the number of electrons donated to it by the ligands is called effective atomic number. It can be calculated as follows:-
EAN of metal in a complex = At. No. of metal – oxidation state + 2 × coordination number of metal.
This number was put forward by Sidgwick to explain the stability of complexes. A stable complex is formed if the EAN is equal to the atomic number of the next noble gas.
Nomenclature of coordination compounds
There are certain rules for writing the name of coordination compounds. They are followings:
1. The positive ion or cation whether simple or complex is named first followed by the anion.
2. The name is started with a small letter and the complex part is written as one word.
3. The name of complex part is written in the following way.
If the complex is cationic or neutral, name of ligands in alphabetical order + name of metal + oxidation no. in Roman digit inside the parenthesis. For example [Co(NH3)4 Cl2]+ is written as tetraamminedichloridocobalt(lll) ion.
If the complex is anionic, pattern is same but metal name is suffixed by ate. For example for Fe, its name is written as ferrate, similarly for Copper is cupperate, for Nickel is nickelate and so on.
4. For several ligands of same type, the prefixes di, tri, tetra are used to indicate the number of ligands.
5. For ligands which have already numerals in their name, their numbers is indicated by bis for 2, tris for 3, tetrakis for 4 and so on.
6. The name of ligands carrying numerals in their name is written inside the small brackets.
7. When the complex is an acid, the name of the metal is made to end with ‘ic’.
Writing formula for coordination compounds:
1. Formula of the cation whether simple or complex is written first followed by anion.
2. The formula of the entire coordination entity whether charged or not is written in square brackets.
3. Within the coordination sphere, the symbol of the metal is written first followed by the symbol or formula of the ligands arranged in alphabetical order. Formulas of ligands are written inside small brackets with their number outside the bracket.
4. The number of cations and anions to be written in the formula is calculated on the basis that total positive charge must be equal to the total negative charge. For example formula of Potassium hexacyanoferate(ll) = K4[Fe(CN)6].
Isomerism in Coordination Compounds
This is the phenomenon in which two or more compounds have the same molecular formulas but different structural or spatial arrangements is called isomerism. These compounds are called isomers. Like organic compounds, there are also two types of isomerism.
1. Structural isomerism and
2. Stereo isomerism
Structural isomerism. This type of isomerism is arises due to difference in structure of coordination compounds. It has been further subdivided into four types. They are followings:
1. Ionisation isomerism. Compounds which give different ions in solution while they have same composition Ionisation isomers and this isomerism is called Ionisation isomerism. This isomerism occurs when counter ions of the complex is also a potential ligand. For example [Co(NH3)5Br]SO4 and Co(NH3)5SO4]Br.
2. Solvate or Hydrate isomerism. Compounds which have the same composition but differ in the number of solvent molecules present as ligands and as free solvent molecules in the crystal lattice are called solvate isomers. In of water solvent, these are called hydrate isomers. For example.
[Cr(H2O)6]Cl3 —- violet
[Cr(H2O)5Cl]Cl2.H2O —- Blue green
Cr(H2O)4Cl2]Cl.2H2O — Dark green
3. Linkage isomerism. This type of isomerism occurs in compounds containing ambidentate ligands. For example. Cr(H2O)5(SCN)]2+ and Cr(H2O)5(NCS)]2+.
4. Coordination isomerism. This type of isomerism is possible when both positive and negative ions of a salt are complex ions and the two isomers differ in the distribution of ligands in the cations and anions. For example. [Co(NH3)6][Cr(CN)6] and [Cr(NH3)6][Co(CN)6]
Stereo isomerism. The isomerism which arises due to the difference in position and arrangement of ligands in space around the metal ion is called stereo isomerism. It is of two types:
1. Geometrical isomerism
2. Optical isomerism
Geometrical isomerism . This isomerism occurs in Heteroleptic complexes due to different possible geometric arrangements of the ligands.
- When two identical ligands occupy adjacent positions, this isomer is called cis- isomer and when arranged opposite to one another, this isomer is called trans-isomer.
- This isomerism is not possible for complexes with coordination number 2, 3 and tetrahedral complexes with coordination number 4 because in these cases, all the positions of ligands are equivalent.
- Cis-trans isomerism is quite common in square planar and octahedral complexes.
- Square planars having general formulas MA2B2, M(AB)2, MA2BC and MABCD types show cis-trans isomerism.
- MABCD types square planar complexes form three isomers. Two of these are cis and any one is trans.
- Octahedral complexes having general formulas MA4B2, MA2B4, M(AA)2B2, M(AA)2BC and MA3B3 show cis-trans isomerism.
- In case of MA3B3 type, cis form is called facial or fac-isomer and tran form is called meridonial or mer- isomers.
Optical isomerism. That isomerism in which isomers rotate the plane polarised light in opposite directions is called optical isomerism. These isomers are called Optical isomers.
- These two optical isomers are structurally non superimposable mirror image of each other.
- They do not possess the plane of symmetry.
- Those optical isomers which rotate the plane polarised light in right directions are called dextrorotatry or d-form isomer.
- Those optical isomers which rotate the plane polarised light in left directions are called laevorotatry or l-form isomer.
- The d and l isomers of a compound are called enantiomers.
- Optical isomerism is common in octahedral complexes with coordination number six having 1, 2 or 3 didentate ligands.
- The octahedral complexes having general formulas [M(AA)3], [M(AA)2B2], [M(AA)2BC] and [M(AA)B2C2] types.
Werner’s theory of Coordination compounds
Alfred Werner was the first scientist to study the bonding in coordination compounds. This theory is known as Werner’s theory of coordination compounds. The main postulates of this theory are followings:
1. In coordination compounds, metals possess two types of valencies known as primary valency and secondary valency.
2. Primary valencies are satisfied by negative ions and secondary valencies are satisfied by ligands.
3. Primary valency represents oxidation state of metal while secondary valency represents coordination numbers.
4. The ions satisfying primary valency are non- directional and the atoms or ions satisfying the secondary valencies are directional and have definite geometry in space.
Determination of the structure of a complex on the basis of Werner ‘s theory.
1. Primary valencies in a coordination compound is represented by dotted lines which are ionisable and non directional .
2. Secondary valencies or linkage of ligands are represented by solid lines.
3. The solid and dotted lines represent the ligands which are similar to counter ions or primary valencies.
Limitations of Werner’s theory. Though Werner’s theory was able to explain a number of properties of the coordination compounds but still could not answer the following questions:
1. Why only certain elements form coordination compounds?
2. Why the coordination sphere has a definite geometry?
3. Why do these compounds possess definite magnetic and optical properties?
Valence Bond Theory in Coordination compounds
This theory was developed by Pauling. The following are the main postulates of this theory.
1. In coordination compounds, the metal- ligand bond arises by the donation of pairs of electrons by ligands to the metal atom or ion.
2. In order to accommodate these electrons, the metal ion must possess required number of vacant orbitals of equal energy and these orbitals are obtained by hybridisation of the orbitals of the metal atom to give a set of hybrid orbitals of equal energy.
3. Sometimes, the unpaired (n – 1) d electrons pair up as fully as possible prior to hybridisation thus making some more orbitals vacant.
4. The central metal atom then makes available the number of empty orbitals equal to its coordination number for the formation of coordinate bonds with suitable ligand orbitals.
5. With the approach of the ligands, metal- ligand bonds are formed by the overlap of these orbitals with those of the ligands.
6. Octahedral, square planar and tetrahedral complexes are formed as a result of d2sp3 or sp3d2, dsp2 and sp3 hybridisation respectively.
Types of octahedral complexes:
There are two types of octahedral complexes.
1. Outer orbital complex. This is also known as high spin complex. This complex is formed when outer nd- orbitals are involved for sp3d2 hybridisation. For example [FeF6]3-, [Ni(H2O)6]2+.
2. Inner orbital complex. This complex is formed when (n – 1) d orbitals are involved in hybridisation sp3d2 to form octahedral complexes. This complex is also known as low spin complex. For example [Co(NH3)6]3+, [Co(CN)6]3-
Limitations of Valence Bond theory
VBT successfully explains the shapes or geometry and magnetic behaviour of the coordination compounds but it has some drawbacks. Which are followings:
1. It can not explain why some complexes of a metal ion in a particular oxidation state are low spin while some other complexes of the same metal ion in same oxidation state are high spin. For example [Co(NH3)6]3+ is a low spin while [CoF6]3- is a high spin complex.
2. The magnetic behaviour predicted by Valence Bond Theory is sometimes misleading. For example, the square planar complex of Ni(ll) should be diamagnetic for dsp2 hybridisation but practically it was found paramagnetic.
3. It could not give any satisfactory explanation for the colour of the complexes.
4. It does not give an exact explanation of thermodynamic or kinetic stabilities of coordination compounds.
5. It does not distinguish between strong and weak ligands.
6. In a number of cases, the magnetic moment observed experimentally do not exactly coincide with the value calculated from valence bond theory.
Crystal Field Theory in Coordination compounds
This theory was developed by Hans Bethe and John Van Vleck. According to this theory:
1. In coordination compounds, the bond between the metal ion and the ligands is ionic arising purely from electrostatic interaction.
2. These interactions are similar to those between the ions in a crystal, that is why it has been named as crystal field theory (CFT).
3. If the ligand is an anion, then the metal ion is a cation.
4. The arrangement of the ligands around the central metal ion is such that the repulsion between these negative points are minimum.
5. In a free transition metal ion, all the five d-orbitals have equal energy and they are degenerate. This degeneracy is maintained if the negative charges present around the central metal atom or ion form a spherically symmetrical field.
6. This field no longer remains symmetrical and the degeneracy is split. The pattern of splitting depends upon the nature of crystal field exerting its effects on the central metal atom or ion.
Crystal field theory for octahedral complexes: As the ligands approach the metal ion, there repulsion between the ligands and d-orbitals, thereby raising their energy relative to that of the free ion. If the d-orbitals are present in a spherically symmetrical crystal field. The mean value of energy of these perturbed d-orbitals is taken as zero and called the Bari centre. Due to difference in orientation of d-orbitals, these orbitals split into two sets of orbitals. The set of orbitals (dx2-y2, dz2) having higher energy (0.6Δ0) is called eg-set and the set of orbitals (dxy, dxz and dyz) having lower energy(- 0.4 Δo) is called t2g- set.
Crystal field theory for Tetrahedral complexes.
In this type of complexes, the d-orbitals of metal atom or ion also split into two sets like octahedral complexes but t2g-set orbitals have higher energy (0.4Δt) and eg-set orbitals have lower energy (- 0.6 Δt).
Crystal field stabilization energy (CFSE). The difference of energy between the two sets of d-orbitals is called CFSE. It is usually denoted by Δo.
Spectrochemical series: The arrangement of ligands in order of their CFSE is called spectrochemical series. They are followings:
I– < Br– < SCN– < Cl– < F– < OH– < C2O4 2- < O2- < H2O < NCS– < NH3 < en < NO2– < CN– < CO
Pairing energy. The energy required for electron pairing in a single orbital is called pairing energy. It is denoted by P.
If Δo < P for the formation of a complex, we get high spin complex and in that case ligands are weak field.
If Δo > P for the formation of a complex, we get low spin complex and in that case ligands are strong field.
Magnetism in coordination compounds. In a coordination compound, if central metal atom or ion has unpaired electrons in its orbitals, it is paramagnetic. If central metal atom or ion has all the paired electrons in its orbitals, it is diamagnetic.
Colour in coordination compounds
If only paired electrons are present in the orbitals of central metal atom or ion in a complex, this compound is colourless. But in case the compound has unpaired electrons, this compound is coloured. Different complexes exhibit different colours due to the ligands attached to central metal atom or ion are different.
Factors affecting the stability of a complex ion
1. Greater the charge on the central metal ion, greater is the stability of the complex.
2. The stability of the divalent metal ions of the first transition series is in the following order:
Mn(ll) < Fe(ll) < Co(||) < Ni(||) < Cu(||) < Zn(||).
3. Greater the basic strength of the ligand, greater is the stability of the complex.
4. Presence of the chelate rings increases the stability of complex.
5. If the ligand happens to be multibidentate and cyclic without any steric effect, the stability of the complex further increased.
6. The stability constant (β) is directly proportional to the stability of the complex.
Organometallic Compounds. Those compounds which contain at least one metal-carbon bond is called organometallic compounds. For example Grignard reagent, diethyl zinc, nickel tetrcarbonyl.
Types of organometallic compounds: Based on the nature of metal-carbon bond, they are broadly classified into two types as followings:-
1. σ- bonded organometallic compounds. This types of compounds are of s and p block elements with M – C σ-bonds. For example Grignard reagent.
2. π- bonded organometallic compounds. This type of complexes are formed by transition elements. For example Zeise’s salt.
Metalcarbonyls. The organometallic compounds in which carbon monoxide acts as the ligands is called metalcarbonys. For example [Ni(CO)4], [Fe(CO)5] etc.
Bonding in metalcarbonyls.
The metal-carbon bonds in metalcarbonyls have both σ and π character. The first overlap takes place between the filled bonding π 2p orbital of carbon monoxide with an empty d-orbital resulting in a σ bond between the metal and carbon atom of carbon monoxide. Here, donation of lone pair of electrons on carbon into a vacant d-orbital of the metal take place.
The second overlap takes place between filled metal d-orbital with an empty antibonding π2p* orbital of carbon monoxide resulting in additional π bond between the metal and same carbon monoxide molecule. The effect of σ bond formation strengthens the π bond and vice-versa. This is called synergic effect. CO and NO are capable to behave as π- acceptor or π-acid ligands.
Summary of coordination compounds notes for class 12th
In this coordination compounds notes for class 12th, we have discussed all the relevant topics which have been prescribed by Ncert or cbse syllabus. We have written about what is coordination compounds, their types, nomenclature. Isomerism. Important bond theories regarding formation of coordination compounds like Werner’s theory, valence bond theory, crystal field theory and many more. We have explained organometallic compounds and their types along with the factors which affect the stability of coordination compounds. We hope that you must have read this notes up to the last. We shall have a humble request to share this notes among your friends and favourites. Thank you.
FAQ in coordination compounds notes for class 12th
Q.No1.What are coordination compounds class 12?
Ans:- Coordination compounds are a type of addition compounds of two or more simple compounds which exist in solid state as well as in solution. This compound is formed between central metal atom and ligands by coordinate bonds.
Q.No2. Which chapter is Werner’s theory class 12?
Ans:- Werner’s theory is related to the coordination compounds chapter 5th of class 12th at present. This is the part of inorganic chemistry.
Q.No3. What is the EAN rule?
Ans:- The full form of EAN is Effective atomic number. It states that a compound is thermodynamically stable when this number is equal to the electrons or atomic number of nearest inert gas.
Q.No4. Who is the father of coordination chemistry? Ans:- Alfred Werner is known as the father of coordination chemistry.
Q.No5. What is primary valency?
Ans:- primary valency represents the oxidation state of central metal atom in coordination compound. This is equal to the number of negative ions attached with central metal atom.

