What are called hydrocarbons?
Those organic compounds which are made of only carbon and hydrogen atoms are called Hydrocarbons. For example, Methane (CH4), Benzene (C6H6). Hydrocarbon Class 11 is very important for Neet, Jee Mains and IIT advance exams.
Hydrocarbons Class 11: Short and Easy Notes
How many types are there of hydrocarbons?
There are four types of hydrocarbons. They are followings:
Alkane, Alkene, Alkyne and Benzene
What are called alkanes ?
Those hydrocarbons which have open chain structure and contains only single bonds between any two carbon atoms are called alkanes. For example Methane, Ethane, Propane, n-butane, Isobutane etc.
Isomerism in alkanes
Alkanes show only two types of isomerism. they are chain isomerism and conformational isomerism
Chain isomerism in Alkanes. Any alkane should have at least 4 carbon atoms to show chain isomerism. For example Methane, Ethane and Propane does not show chain isomerism. Butane has 2 chain isomers. Pentane has 3 chain isomers.
Conformational Isomerism in Alkane.
This isomerism arises due to free rotation of σ-bonds around carbon – carbon single bond.
Conformational isomerism in ethane. In ethane, this isomerism can be represented by two methods: 1. Sawhorse formulae 2. Newman projection formulae
1. Sawhorse Formulae. This is a simple method of representing three dimensional formulae on paper. In this method, the bond between the two carbon atoms is drawn diagonally and is slightly elongated for clarity. The lower left hand carbon is considered to be towards the front and the upper right hand carbon towards the back. Ethane can be represented in two ways as staggered and eclipsed conformation.
2. Newman projection formulae. These projection formulae are obtained by viewing the molecule along the bond joining the two carbon atoms. The carbon atom near the eye is represented by a point and the three atoms or groups[ attached to it by three equally spaced (120o) radii.
The carbon atom farther from the eye is designated by a circle and the three atoms or groups attached to it by the three spaced radial extension. In this method, Ethane can be represented in two ways as staggered and eclipsed conformation. In any method, staggered form is more stable than eclipsed form.
Preparation of Alkanes
1. From unsaturated hydrocarbons. Alkenes and alkynes add one and two molecules of hydrogen respectively in presence of a catalyst such as Raney nickel, platinum or palladium to form alkanes.
2. From alkyl halides. Alkyl halides except fluorides on reduction with zinc and dilute hydrochloric acid give alkanes. Alkyl halides on treatment with sodium metal in dry ethereal solution give higher alkanes and this reaction is called Wurtz reaction.
3. From carboxylic acid. Carboxylic acid can be converted into alkanes in two ways.
1. By Decarboxylation. Sodium salts of carboxylic acids on heating with sodalime ( a mixture of sodium hydroxide and calcium oxide ) give alkanes one carbon atom less than the carboxylic acid.
2. Kolbe’s electrolytic method. An aqueous solution of sodium or potassium salt of a carboxylic acid on electrolysis gives alkane containing even number of carbon atoms at the anode.
Physical properties of Alkanes
Alkanes are almost non polar molecules. They have weak Vander forces among their molecules. Therefore, the first four members are gases. Five to seventeen are liquids and those contain 18 or more carbons are solid. They are colourless and odourless. They have low boiling points. The boiling of alkanes increases with increase in molecular mass. In case of isometric alkanes, their boiling points decreases with increase in branch.
Chemical properties of Alkanes
Alkanes are generally inerts towards most of the chemical substances. However, they undergo the following reactions under certain conditions.
1. Substitution reactions. One or more hydrogen atoms can be replaced by halogens, nitro and sulphonic acid groups. Halogenation takes place either at higher temperature (573 – 773K) or in the presence of diffused sunlight or ultraviolet light. Lower alkanes do not undergo nitration and Sulphonation reactions.
2. Combustion. Alkanes on heating in the presence of air or oxygen are completely oxidized to CO2 and H2O with the evolution of large amount of heat. During incomplete combustion of alkanes with insufficient amount of air or oxygen , carbon black is formed which is used in the manufacture of ink, printer ink, black pigments as filters.
3. Controlled oxidation. Alkanes on heating with regulated supply of air or oxygen at high pressure and in the presence of suitable catalysts give a variety of oxidation products like alcohol, aldehyde and carboxylic acid.
4. Isomerisation. n-Alkane on heating in the presence of aluminium chloride and hydrogen chloride gas isomerise to branched chain alkanes. For example n-hexane isomerises into 2- methyl pentane and 3- methyl pentane.
5. Aromatization. n- alkane having six or more carbon atoms on heating to 773K at 10-20 atmospheric pressure in the presence of oxides of aluminium. molybdenum or chromium supported over alumina get dehydrogenated and cyclysed to benzene and its homologous. This reaction is known as aromatization.
6. Pyrolysis. Higher alkanes on heating to higher temperature decompose into alkanes and alkenes of less carbon atoms. This process is called pyrolysis.
Alkene
This is the hydrocarbons of open chain which must contain at least one double bond. For example ethene, propene etc.
Isomerism in Alkene
Alkenes show structural isomerism and geometrical isomerism.
Structural isomerism. Alkenes show two types of structural isomerism as chain isomerism and position isomerism. For example. In case of butene (C4H8), but-1-ene and but-2-ene are position isomers and but-1-ene or but-2-ene and 2-methyl prop-1-ene are chain isomers.
Geometrical isomerism. Double bonded carbon atoms have to satisfy the remaining two valencies by joining with two atoms or groups. The two atoms or groups attached to each carbon atoms are different. They can be represented by YXC = CXY like structure. YXC = CXY can be represented in the space by two ways as cis-form and trans-form.
- Cis-form. In this representation, the same atoms or groups along double bonded carbon atoms are in same direction. These isomers have high boiling points, dipole moment with respect to trans isomers. They are polars.
- Trans-form. In this representation, the same atoms or groups along double bonded carbon atoms are in opposite direction. The are generally non-polar but have higher melting point than cis-forms.
Preparation of Alkenes
1. From alkynes. Alkynes on partial reduction with calculated amount of dihydrogen in the presence of palladised charcoal partially deactivated with poisons like sulphur compounds or quinoline give alkene. Partially deactivated palladised charcoal is known as Landler’s catalyst. Alkenes thus obtained are having cis geometry. However, alkynes on reduction with sodium in liquid ammonia form trans alkenes.
2. From alkyl halides. Alkyl halides (R – X) on heating with alcoholic potash eliminate one molecule of alkyl halide into alkene. This is an example of B-elimination reaction. This reaction is also known as dehydrohalogenation.
3. From vicinal dihalides. Dihalides in which two halogen atoms are attached to two adjacent carbon atoms are known as vicinal dihalides. These dihalides on treatment with Zn metal loss a molecule of ZnX2 to form an alkene. This reaction is known as dehalogenation.
4. From alcohols by acidic dehydration. Alcohols on heating with conc.H2SO4 form alkenes with the elimination of one molecule of water. This reaction is also an example of β-elimination.
Physical properties of alkenes
The first three members are gases. The next fourteen are liquids and the higher ones are solid. Ethene is a colourless gas with a faint sweet smell. All other alkenes are colourless and odourless. They are insoluble in water but fairly soluble in non- polar solvents like benzene, petroleum, ether. They show regular increase in boiling point with increase in size. Like alkane, straight chain alkenes have higher boiling point than isomeric branched chain alkenes.
Chemical properties of Alkenes
Alkenes are the rich source of loosely held π- electrons, due to which they show addition reaction in which the electrophiles add on to the C = C to form the addition product. Under Special conditions, alkenes alkenes also undergo free radical substitution reactions. Oxidation and ozonolysis are also quite prominent in alkenes. Important reactions of alkenes are followings:
1. Addition of dihydrogen. Alkenes add up one molecule of dihydrogen gas in the presence of finely divided nickel, palladium or platinum to form alkanes.
2. Addition of halogens. Halogens like bromine or chlorine add up to alkene to form vignal dihalides. However, Iodine does not show addition reaction under normal conditions. The reddish brown colour of bromine solution in carbon tetrachloride is discharged when bromine adds up to an unsaturation site. This reaction is used for the test of unsaturation.
3. Addition of hydrogen halide. Hydrogen halides (HCl, HBr, HI) add up to alkene to form alkyl halides. The order of reactivity of the hydrogen halide is HI > HBr > HCl.
Markovnikov’ rule. This rule is applied in addition of hydrogen halide with unsymmetrical alkenes. This rule states that negative part of adding molecules gets attached to that carbon atom which possesses less number of hydrogen atoms. For example, addition of HCl to propene gives 2- chloro propane as major product.
Anti Markovnikovs Rule. In the presence of peroxide, addition of HBr to unsymmetrical alkenes like propene takes place contrary to the Markovnikov’s Rule. This happens only with HBr but not with HCl and HI. This reaction is also known as kharash effect or peroxide effect. For example, Addition of HBr with propene in the presence of benzoyl peroxide gives 1-bromo propane as major product.
4. Addition of Sulphuric acid. Cold concentrated H2SO4 adds to alkenes accordance with Markovnikov’s Rule to form alkyl hydrogen sulphate by the electrophilic addition reaction.
5. Addition of Water. In the presence of a few drops of conc. H2SO4 alkenes react with water to form alcohols in accordance with Markovnikov’s Rule .
6. Oxidation. Alkenes on reaction with cold, dilute aqueous solution of potassium permanganate (Bayer’s reagents) produce vicinal glycols. Decolorisation of KMnO4 solution is used as a test for unsaturation.
Acidic Potassium permanganate or acidic Potassium dichromate oxidises alkenes to ketones and/or acids depending upon the nature of alkanes and the experimental conditions. For example but-2-ene produces two moles of ethanoic acid.
7. ozonolysis. Ozonolysis of alkenes involves the addition of ozone molecule to form ozonide, and then cleavage of the ozonide by Zn-H2O to smaller molecules. This reaction is highly useful in detecting the position of the double bond in alkenes.
8. Polymerisation. A large number of alkenes at high temperature, high pressure and in the presence of a suitable catalysts form bigger molecules. This process is called polymerisation. For example, the formation of Polythene from ethene.
Alkynes
The open chain hydrocarbons which have at least one triple bond in their chains are called alkynes. Its general formula is CnH2n-2. For example, acetylene, propyne, butane etc.
Isomerism in alkynes
Alkynes show structural isomerism.
An alkyne having carbon atoms more than three can show two types of structural isomerism. They are chain isomerism and position isomerism. For example, an alkyne having formula C4H6 can be represented as but-2-yne and but-1-yne as position isomers whilebut-2-yne or but-1-yne and 2- methyl propyne as chain isomers.
Preparation of Alkynes
1. From calcium carbide. On industrial scale, ethyne is prepared by treating calcium carbide with water.
CaC2 + H2O —– Ca(OH)2 + C2H2
2. From vicinal dihalide. Vicinal dihalide on treatment with alcoholic Potassium hydroxide undergo dehydrohalogenation and form alkenyl halide which further on treatment with sodamide gives alkyne.
Physical properties of Alkynes
Like alkanes and alkenes, the first three members of alkynes are gases, the next eight are liquids and the higher ones are solids. All alkynes are colourless. Except ethynes other members are odourless. They are lighter than water and immiscible with water but soluble in organic solvents like ethers, carbon tetrachloride and benzene. Their melting point, boiling point and density increases with increase in molar mass.
Chemical properties of Alkynes
Alkynes show acidic nature, addition reactions and polymerisation reactions as follows :
1. Acidic character of Alkynes
Sodium metal and sodamide are strong bases. They react with acetylene to form sodium acetylide with the liberation of hydrogen gas. This reaction justifies that acetylene or ethyne is acidic in nature. Order of acidic strength in alkynes follow as:
CH ≡ CH > CH3 – C ≡ CH > CH3 – C ≡ C – CH3.
2. Addition reactions. Alkynes contain a triple bond. So they add up two molecules of dihydrogen, halogen, hydrogen halide etc. A few addition reactions are given below:
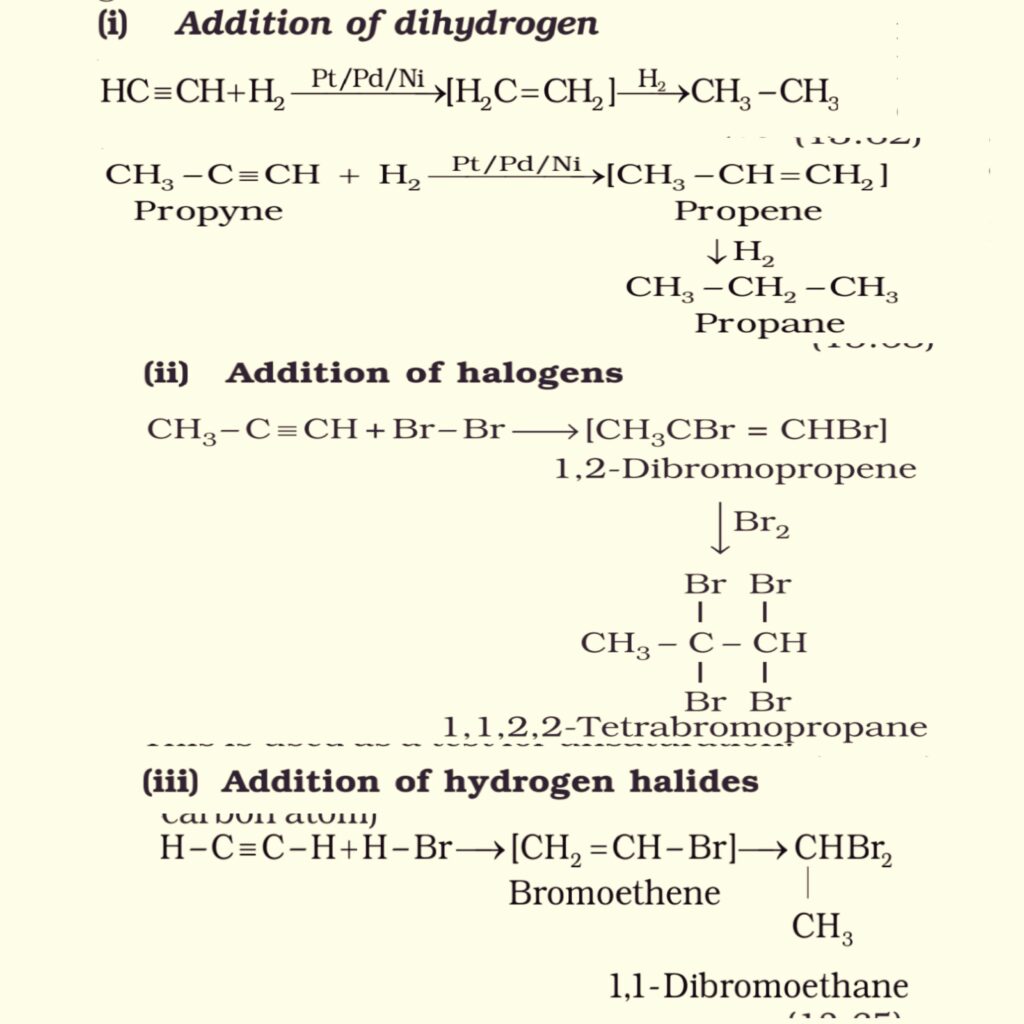
3. Polymerisation. There are two types of polymerisation in alkynes.
1. Linear polymerisation. Under suitable conditions, linear polymerisation of ethyne takes place to produce polyethyne. This polymer conduct electricity and have high molecular mass.
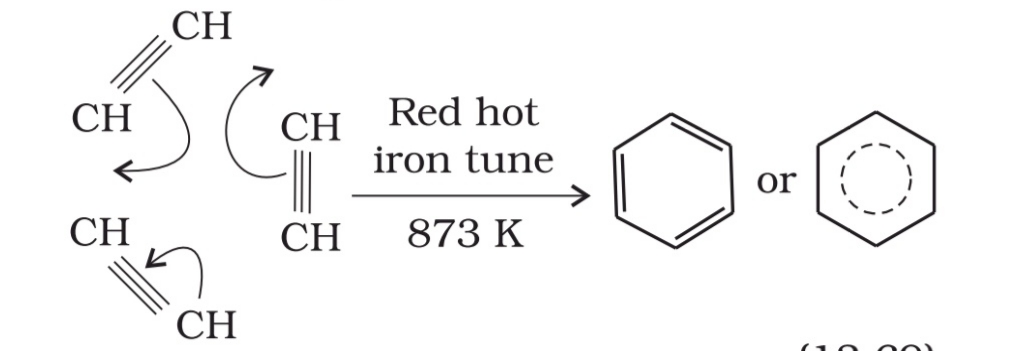
2. Cylic polymerisation. Ethyne or acetylene passing through a red hot Cu-tube at 873K undergoes cylic polymerisation and form benzene. This is the best route for entering from aliphatic to aromatic compounds.
Aromatic hydrocarbons
These hydrocarbons possess pleasant smell hence they are called aromatic hydrocarbons. Most of such compounds were found to have benzene rings. They are also known as arenes. Benzene ring is highly unsaturated. Aromatic compounds having benzene rings are called benzenoid. Some aromatic compounds do not contain benzene rings are called non- benzenoid.
Structure of benzene
Benzene was isolated by Michael Faraday’s in 1825. Benzene was found to form triozonide on ozonolysis which indicates the presence of three double bonds in benzene. Benzene was further found to form one and only one mono substituted derivatives which indicates that all the six carbon atoms and all the six hydrogen atoms benzene are identical. On the basis of this observation August Kekule proposed the structure of benzene that it has cylic arrangement of six carbon atoms with alternate single and double bonds and one hydrogen atom attached to each carbon atom.
Resonance and stability of benzene
According to valence bond theory, the structure of benzene can be represented by two ways and these structures are called resonating or contributing structures. No one structure of them is sufficient to explain all the properties of benzene. Hence the hybrid structure of benzene was proposed and this structure is known as resonance hybrid. This structure is represented by inserting a circle or a dotted circle in the hexagon. The circle represents the six electrons which are delocalised between the six carbon atoms of the benzene ring.
Aromaticity. Benzene was considered as parent ‘aromatic’ compounds. This name is applied to all the ring systems weather or not having benzene rings. The following characters are possessed by them.
• Planarity
• Complete delocalisation of the # electrons in the ring.
• Presence of (4n + 2) π electrons in the ring. Where n = 0, 1, 2, 3 —–. This regulation is known as Huckel rule.
Preparation of Benzene
1. By cyclic polymerisation of ethyne. Already discussed.
2. By decarboxylation of aromatic acids. Sodium salt of benzoic acid on heating with sodalime gives benzene.
3. By reduction of Phenol. Phenol is reduced to benzene by passing its vapours over heated zinc dust.
Physical properties of Benzene
Aromatic hydrocarbons are non- polar molecules and are usually colourless liquids or solids with characteristic smell. Aromatic hydrocarbons are immiscible with water but readily soluble in organic solvents. They burn with sooty flames.
Chemical properties of benzene
Aromatic compounds are generally involved in electrophilic substitution reactions. However, under special conditions they can also undergo addition and oxidation reactions.
1. Electrophilic substitution reactions. The common electrophilic substitution reactions in arenes are nitration, sulphonation, halogenation and friedel’s craft reactions
A. Nitraton. A nitro group is introduced into benzene ring when benzene is heated with a mixture of concentrated Nitric acid and sulphuric acid.
B. Halogenation. Arenes react with halogens in the presence of Lewis acid like anhydrous FeCl3, FeBr3 or AlCl3 to yield haloarenes.
C. Sulphonation. The replacement of a hydrogen atom by a sulphonic acid group is called Sulphonation. It is carried out by heating benzene with fuming Sulphuric acid (oleum).
D. Friedel crafts alkylation. When benzene is treated with an alkyl halide in the presence of anhydrous aluminium chloride, alkyl benzene is formed.
E. Friedel crafts acylation. The reaction of benzene with acyl halide or acid anhydride in the presence of Lewis acid AlCl3 produce acyl benzene.
2. Addition reactions. Benzene gives cyclohexane when it is treated with dihydrogen in the presence of nickel catalyst at high temperature and/or pressure after hydrogenation.
Under ultraviolet light, three chlorine molecules add to benzene to produce benzene hexachloride, C6H6Cl6 which is also called gammaxane.
3. Combustion. When benzene is heated in air, it burns with sooty flame producing CO2 and H2O.
Directive influence of functional group in monosubstituted benzene
When monosubstituted benzene is subjected to further substitution, two types of behaviour are observed. Either ortho and para or meta products are predominately. This behaviour depends on the nature of the functional group already present in the benzene ring.
1. Ortho and para directing groups: The groups which direct the incoming groups towards ortho and para positions are called ortho and para directing groups. For example, -OH, -NH2, -NHR, – OCH3, – CH3 etc.
2. Meta directing groups: The groups which direct the incoming groups towards meta positions are called meta directing groups. For example, -NO2, – CN, – CHO, – COOH, -COR, – COOH, – SO3H etc.

