Ionic equilibrium is the state of an Ionic reaction in which dissociated ions and undissociated Ionic compounds (electrolytes) are in equilibrium state. We shall study in this chapter about strong and weak electrolyte, Ionisation of acids and bases, degree of ionization, concept of pH, hydrolysis of salt, buffer solution, solubility product and common ion effects with suitable examples. This chapter is very interesting and important for the entrance exams of Jee(mains), NEET, IIT advance and other competitive exams. Let’s start from here.
Important terms of Ionic Equilibrium
Electrolyte : Those compounds whose aqueous solution or melt conducts electricity and dissociate into ions are called electrolytes. For example NaCl, CH3COOH, KOH etc.
Types of electrolytes. On the basis of Degree of dissociation, there are two types of electrolytes.
1. Strong electrolyte. Those electrolytes which completely dissociate into ions in its solution or melt are called strong electrolytes. Degree of dissociation for strong electrolytes is one. Example strong acid, strong base and salts.
2. Weak electrolyte. Those electrolytes which partially dissociate into ions in its solution or melt are called weak electrolytes Degree of dissociation for strong electrolytes is less than one. Example weak acid and weak base.
Degree of dissociation. The fraction of the total number of molecules which dissociates into ions is called the degree of dissociation or degree of ionization. It is usually represented by α (alpha).
α = No. of moles dissociated/ Total no. of moles taken.
Ostwald’s Dilution Law
This law was given w.r.t. the Ionisation of weak electrolytes. According to this law, in case of a weak electrolyte the degree of ionization is inversely proportional to the square root of molar concentration or directly proportional to the square root of Volume containing one mole of solute.
α = √Ka/C and α = √Ka.V
Various concepts of Acids and Bases
Classical concept of Acids and Bases
Acid. Chemical substances whose aqueous solution has the following characteristic properties are called acids.
- Conducts electricity,
- Reacts with active metals like zinc, magnesium etc.to give hydrogen.
- turns blue litmus red.
- Has a sour taste.
- Whose acidic properties disappear on reaction with a base.
Base. Chemical substances whose aqueous solution has the following characteristic properties are called bases.
- Conducts electricity.
- Turns red litmus blue.
- Has a bitter taste.
- has a soapy (slippery) touch.
- Whose basic properties are destroyed on reaction with an acid.
Arrhenius Concepts of acids and Bases
Acid. A chemical substance which gives hydrogen ion (H+) on dissolution in water is called acid. For examples HCl, HNO3, H2SO4 etc.
Base. A chemical substance which gives hydroxyl ion (OH-) on dissolution in water is called bases. For examples NaOH, KOH, NH4OH etc.
Limitation of Arrhenius Concepts.
- Inability to explain the existence of hydrogen ions and hydroxyl ions in the aqueous solution.
- Inability to explain acidic and basic character of certain substances like NH3, Na2CO3, CaO, CO2, SO3 etc.
- Inability to explain the reaction between an acid base in absence of water.
NH3 (g) + HCl (g) → NH4Cl(s)
3. Bronsted- Lowry Concept of Acids and Bases
A chemical substance which has tendency to give a proton (H+) is called acid and which has tendency to accept a proton is called a base. Some important examples are followings:-
HCl + H2O ⇌ H3O+ + Cl–
(Acid)
NH3 + H2O ⇌ NH4+ OH–
(base)
Conjugate pair of acid and base:
A pair of acid and base which have difference in their formula or composition by H+ ion is called a conjugate pair of acid and base.
Conjugate acid ⇌ Conjugate base + H+
Examples are followings:-
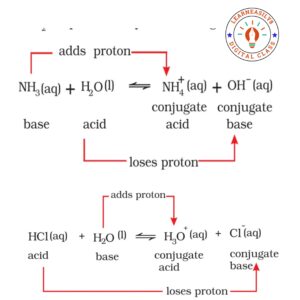 Limitations of Bronsted- Lowry concept
Limitations of Bronsted- Lowry concept
1. It cannot explain the reactions between acidic oxides like CO2, SO2, SO3 etc. and the basic oxides like CaO, BaO, MgO etc.
2. Substances like BF3, AlCl3 etc. do not have any hydrogen and hence cannot give a proton but they behave like acids.
4. Lewis Concepts Acids and Bases.
An acid is defined as substance (atom, ion or molecule) which is capable to accept a pair of electrons and a base is defined as a substance which is capable to donate a lone pair of electrons. In short, an acid is an electron pair acceptor and a base is an electron donar.
Types of Lewis bases. Lewis bases can be of two types.
1. Neutral molecules like NH3, R-NH2, R-OH, H-O-H etc.
2. All negative ions like F–, Cl–, OH–, CN– etc.
Types of Lewis acids. Lewis acids can be of four types.
1. Molecules having a central atom with incomplete octet. Ex. BF3, AlCl3, FeCl3.
2. Simple cations like Ag+, Cu2+, Fe3+,
3. Molecules having central atoms with empty d-orbitals example SnCl4, SiF6, PF5 etc.
4. Molecules containing a multiple bond between two atoms of different electronegativity. Example O = C = O.
READ MORE: Chemical Equilibrium for Class 11th
Strength of Acids and Bases
Strength of Acids. As we know that there are of two types of acids. They are hydra acids and oxa acids.
1. Strength of hydra acid (Hn Xn)
(a) When O.N. of X is same, The strength of hydra acid α the size of X-atom.
for example: HF < HCl < HBr < HI
(b) When X-atoms have approximately same size, O.N. of X-atom α The strength of acid.
For example: CH4 < NH3 < H2O < HF where oxidation no. of C, N, O and H are -4, -3, -2 and -1 respectively.
2. Strength of Oxa acids (HO-X). Examples are H2SO4, HNO3, H3PO4 etc.
(a) O.N. of X-atom α Strength of acid.
For example: HClO < HClO2 < HClO3 < HClO4
(b) When O.N. of the X-atom is same, The size of X-atom α 1/ strength of acid.
For example: HClO4 > HBrO4 > HIO4, where the size of Cl > Br > I.
(c) Relative charge on O-atom in Oxa acid α Strength of acids.
For example: HClO4 > HNO3 > H2SO4 > H3PO4. as relative charge on oxygen atom in HClO4, HNO3, H2SO4 and H3PO4 are respectively are – 0.25, -0.33, -0.5 and -0.75 respectively.
Strength of Bases.
1. Size of metal present in a base ∝ the strength of base
For example: LiOH < NaOH < KOH < RbOH
2. Oxidation number of metal ∝ 1/strength of base.
For example: NaOH > Ca(OH)2 > Al(OH)3, where O.N. of Na, Ca and Al are +1, +2 and +3 are respectively.
⇒ Dissociation constant of acid and base α strength of acid or base
i.e. Ka α strength of acid where Ka is dissociation constant of base.
and, Kb α strength of base where Kb is dissociation constant of base.
⇒ Relative strength of two acids or two bases
1. strength of acid 1 / strength of acid 2 = √ Ka1/Ka2 = αa1/αa2
2. strength of base 1 / strength of base 2 = √ Kb1/Kb2 = αb1/αb2
⇒ Strength of Lewis acid and Lewis base
. The Lewis acid character decreases down the group as:
BX3 > AlX3 > GaX3 > InX3 (where X = F, Cl, Br, I)
. The Lewis acid character increases on increase in the size of halogen atoms.
BF3 < BCl3 < BBr3 < BI3
. The basic strength order is following
NH3 > PH3 > AsH3 > SbH3
NF3 < NCl3 < NBr3 < NI3
About PH (potenz de hydrogen)
It is a negative logarithm of hydrogen or hydronium ion concentration. (by Sorensen Sir)
i.e. PH = – log [H+] or [H3O+]
. This value indicates that given solution is acidic, alkaline or neutral.
. If PH < 7, the solution is acidic. at 25oC
. If PH = 7, the solution is neutral. at 25oC
. If PH > 7, the solution is alkaline. at 25oC
Important characteristics of pH
1. If temperature increases, the value of PH decreases.
2. The pH values of the solutions do not give the exact idea of their relative strength. For example:
• A solution of pH = 1 has hydrogen ion concentration 100 times than that of a solution of pH = 3 and not 3 times.
• A 4 ×10-5N HCl solution is twice concentrated as compared to 2×10-5 N HCl solution but the pH values of these solutions are 4.4 and 4.7 respectively and not double.
3. pH = 0 is obtained in 1N solution of a strong acid and for concentration 2N, 3N, 10N etc.the value can be negative too. Similarly for a concentrated solution of a strong base (>1), pH can be greater than 14.
4. A 10-8 M solution of acid cannot have pH = 8 but the value will be close to < 7.
Some important Formulas
pH = – log [H+], pOH = – log [OH–], pKa = – log Ka, pKb = – log Kb pKw = – log Kw
Ionic product of water: (Kw)
Kw = [H+] [OH-] and pKw = pH + pOH
Kw = 10-14M at 25oC and pKw = 14
Relation between Ka and Kb
Ka.Kb = [H+].[OH-], or Ka.Kb = Kw
after applying log both side we shall get pKa + pKb = 14 (pKw)
Hydrolysis of salt:
Reaction of a salt with water to produce corresponding acid and base is called hydrolysis of salt. For example
BA + H2O →BOH + HA
salt base acid
Types of salt and their hydrolysis
Based on the relative strength of radicals (acidic or basic) in salts, there are four types of salts.
1. Neutral salt. A salt of strong acidic radical and a strong basic radical is known as neutral salt. The solution of this type of salt has pH = 7 at 25oC and does not undergo hydrolysis. For example NaCl, K2SO4, KNO3, Na2SO4 etc.
2. Basic salt. A salt of strong basic radical and a weak acidic radical is known as basic salt. The solution of any basic salt has pH > 7 and this type of salt undergo hydrolysis. For example CH3COONa, K2CO3, NaCN, Na3PO4 etc.
3. Acidic salt. A salt of weak basic radicals and strong acidic radicals is known as acidic salt. The solution of any acidic salt has pH < 7 and this type of salt undergo hydrolysis. For example NH4Cl, CuSO4, AlCl3 etc.
4. Salts of weak acid and weak base. For example CH3COONa, (NH4)2CO3, AlPO4. This type of salts also undergo hydrolysis and pH has slightly greater than 7.
Important formulas for hydrolysis of salt.
1. For hydrolysis of basic salt.
▪Determination of hydrolysis of constant (Kh)
and, Kh = Kw/Ka
▪Determination of degree of hydrolysis (h) and h = √Kh/C or h = √Kw/Ka.C
▪Determination of pH
pH = 7 + 1/2(pKa + logC)
2. For hydrolysis of acidic salt.
▪Determination of hydrolysis of constant (Kh)
and, Kh = Kw/Kb
▪Determination of degree of hydrolysis (h) and h = √Kh/C or h = √Kw/Kb.C
▪Determination of pH
pH = 7 – 1/2(pKb + logC)
3. For hydrolysis of weak acid and weak salt.
▪Determination of hydrolysis of constant (Kh)
and, Kh = Kw/Ka.Kb
▪Determination of degree of hydrolysis (h) and h = √Kh or h = √Kw/Kb.Ka
▪Determination of pH
pH = 7 + 1/2(pKa – pKb)
Buffer solution in Ionic Equilibrium
A solution which resists any change in its pH value even when small amounts of the acid or the base are added to it is called buffer solution.
Types of buffer solutions: There are two types of buffer solutions:
1. Solutions of single substances. The solution of the salt of a weak acid and a weak base acts as a buffer solution. For example of the solution of ammonium acetate (CH3COONH4).
2. Solutions of mixtures. These are further of two types:
(a) Acidic buffer. It is the solution of a mixture of a weak acid and a salt of this weak acid with a strong base. For example CH3COOH + CH3COONa.
(b) Basic buffer. It is the solution of a mixture of a weak base and a salt of this weak base with a strong acid. For example NH4OH + NH4Cl.
Calculation of pH of a buffer mixture
(1) For acidic buffer mixture. The equation provided for the calculation of pH for acidic buffer mixture is called Henderson- Hasselbalch equation. Here pH = pKa + log [salt]/[acid] or pH = pKa + log [conjugate base]/[acid].
(2) For basic buffer mixture. Here pOH = pKb + log [salt]/[base] or pOH = pKb + log [conjugate acid]/[base].
Buffer capacity: The ratio between the number of moles of the acid or base added to 1L of the buffer and change in pH is called buffer capacity. Buffer capacity = dn/ dpH.
Solubility Equilibrium and Solubility Product
Solubility product. The product of the molar concentration of the ions of a sparingly soluble salt in a saturated solution, each concentration raised to the power equal to the number of ions produced on dissociation of one molecule of this salt is called solubility product. For example
In general, for any electrolyte AxBy may be written as:
AxBy ⇌ xAy+ + yBx-
Thus, the solubility product for AxBy may be written as
Ksp = [Ay+]x x [Bx-]y
Where X and y represent the number of ions in the formula of the salt or electrolyte.
Common ion effect in Ionic Equilibrium
Suppression in the dissociation of a weak electrolyte by adding a strong electrolyte having a common ion to this weak electrolyte is known as common ion effect. For example if HCl (a strong electrolyte) is added in a solution of H2S gas (a weak electrolyte)
HCl → H+ + Cl–
H2S ⇌ 2H+ S2- Addition of HCl decreases the dissociation of H2S by increasing the concentration of H+ ions which is common.
Application of solubility product and common ions effect
1. In calculation of solubility of a sparingly soluble salt by the relationship b/w solubility and solubility product.
2. In prediction of ionic or precipitation reaction. If the ionic products of ions in the solution exceeds the value of solubility product of a sparingly soluble salt.
3. In explaining salting out or precipitation of soluble salts. precipitation of NaCl from its solution by adding HCl or precipitation of soap by adding NaCl in its solution is called salting out.
4. The separation and identification of various basic radicals into different groups.
- Sulphides of cations (Hg2+, Cu2+, Cd2+, Bi3+,As3+,Sb2+) of group ll are precipitated by H2S gas through acidified solution.
- Sulphides of cations (Co2+, mn2+, Zn2+, Ni2+) of group lV are precipitated by passing H2S gas through ammonical solution of these cations.
- Hydroxide of cations ( Al3+, Fe3+, Cr3+) of group lll are precipitated by adding NH4Cl and NH4OH
5. In fractional precipitation. The solubility is lower, precipitation is easier.
Acid – Base Titration Using indicators
Titration. A technique to determine qualitatively the concentration of one substance if that of the other is known as titration.
Indicator. A chemical substance that is used to determine the end point in a titration is called indicator. Indicators are usually weak organic acid (phenolphthalein) or a weak organic base (methyl orange) which shows the change in Colour with in a certain change in Colour or at the end point of the reaction.
End point. When one equivalence of an acid completely reacts with one equivalence of a base.
Selection of indicators:
1. For the titration of a strong acid vs. strong base, any indicator either acidic (Hph) or basic (MeOH) can be used.
2. For a weak acid against a strong base like NaOH or KOH, only acidic indicator (Hph) can be used.
3. For a weak base against a strong acid like HCl, only basic indicator (MeOH) can be used.
• The indicator selected in such a condition that pKln = pH of the solution. Where pKln = – log Kln and Kln is the dissociating constant of Hph or Hln.
Hln(aq) ⇌ H+(aq) + ln–(aq)
And, Kln = [H+][ln–]/[Hln].
Ostwald’s theory for the use of indicators in titration.
Indicators are a weak organic acid or base which after Ionisation have different colour from their ions.
Example :
Hph ⇌ H+ + ph–
Colourless pink
MeOH ⇌ Me+ + OH–
Yellow red

