Ncert Solution of Electrochemistry: This is the 2nd chapter of chemistry for class 12th according to the revised syllabus of Ncert pattern. This is a wing of physical chemistry. We must learn this chapter for the preparation of class 12th board and other entrance exams like Jee(mains), NEET, IIT advance. We have included the solution of all the questions weather intext or exercise of this chapter. Solution have been written in simple and proper ways so that students can have a good grip on the theory and principles on Electrochemistry chapter.
Ncert Solution of Electrochemistry not only helps to solve the problems of this chapter but also provides the ways following which a student can solve the other problems regarding this chapter. During solution of numericals of this chapter, most of derivations and formulas have been used at advance level. Therefore, you must take help of this solution till end.
The objectives of the Ncert Solution of Electrochemistry:
- To describe an electro chemical cell and differentiate between galvanic electrolytic cell.
- To apply Nernst equation for calculating the emf of galvanic cell and define standard potential of the cell.
- To derive relationship between standard potential of the cell, Gibbs energy of cell reaction and its equilibrium constant.
- To define resistivity (μ), conductivity (κ) and molar conductivity (∧m) of ionic solution.
- To differentiate between ionic and electronic conductivity.
- To describe the method for measurement of conductivity of electrolytic solutions and calculation of their molar conductivity.
- To justify the variation of conductivity and molar conductivity of solutions with change in their concentration and define ∧om (molar conductivity at infinite dilution)
- To explain Kohlrausch law and learn what are its applications.
- To understand quantitative aspects of electrolysis.
- To describe the constructions of some primary and secondary batteries and fuel cells.
- To explain corrosion as an electrochemical process.
Answer of Intext questions in Ncert Solution of Electrochemistry
Question 1. How would you determine the standard electrode potential of the system Mg2+| Mg?
Answer: We shall set up a cell containing Mg | MgSO4 (1M) as one electrode and standard hydrogen electrode Pt, H2 (1 atm) | H+ (1M) as the second electrode and both are connected by voltmetre. Now measure the EMF of the cell and also note the direction of deflection in the voltmeter. The direction of deflection shows that electrons flow from Magnesium electrode to hydrogen electrode that means oxidation takes place on magnesium electrode and reduction on hydrogen electrode.
Hence the cell may be represented in the following way: Mg | Mg2+(1M) || H+(1M) | H2, (1atm), Pt or Eocell = EoH+/1/2H2 – EoMg2+/Mg as EoH+/1/2H2 = 0 hence, Eocell = EoMg2+/Mg.
Question 2. Can you store copper sulphate solution in a zinc pot?
Answer: Zinc is more reactive than copper. Hence, it displaces copper from copper sulphate solution as follows Zn(s) + CuSO4 (aq) → ZnSO4 (aq) + Cu (s). Thus, zinc reacts with CuSO4 solution. Hence, we cannot store Copper sulphate solution in zinc pot.
Question 3. Consult the table of the standard electrode potentials and suggest three substances that can oxidise ferrous ions under suitable conditions.
Answer: Oxidation of ferrous ions means that the following reaction should take place: Fe2+ → Fe3+ + e– ; Eoox = – 0.77V. Only those substances can oxidise Fe2+ to Fe3+ which are stronger oxidising agents and have positive reduction potential greater than 0.77 V so that EMI of the cell reaction is positive. This is so for elements lying below Fe3+/Fe2+ in the electrochemical series, e.g., Br2, Cl2 and F2.
Question 4. Calculate the potential of hydrogen electrode in contact with a solution whose pH is 10.
Answer: For hydrogen electrode, H+ + e– → 1/2 H2. 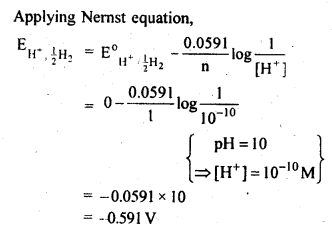
Question 5. Calculate the emf of the cell in which the following reaction takes place:
Ni(s) + 2Ag+ (0.002 M) → Ni2+ (0.160 M) + 2Ag(s), Given that Eo(cell) = 1.05 V .
Answer: 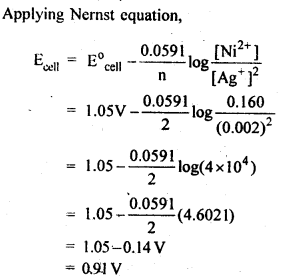
Question 6. The cell in which the following reaction occurs: 2Fe3+ (aq) + 2I– (aq) → 2Fe2+ (aq) +I2 (s) has E°cell= 0.236 V at 298 K. Calculate the standard Gibbs energy and the equilibrium constant of the cell reaction.
Answer: 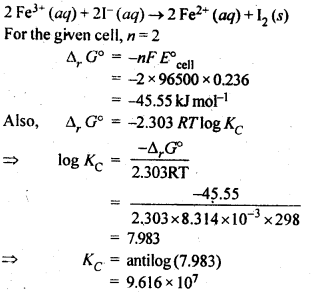
Question 7. Why does the conductivity of a solution decrease with dilution?
Answer: Conductivity of a solution is the conductance of ions present in a unit volume of the solution. On dilution, the number of ions per unit volume decreases. Hence, the conductivity decreases.
Question 8. Suggest a way to determine the ∧om value of water.
Answer: ∧om (H2O) = λoH+ + λoOH– Now we will find out ∧om (NaCl), ∧om (HCl) and ∧om (NaOH). Then ∧om (H2O) = ∧om (HCl) + ∧om (NaOH) – ∧om (NaCl).
Question 9. The molar conductivity of 0.025 mol L-1 methanoic acid is 46.1 S cm2 mol-1. Calculate its degree of dissociation and dissociation constant Given λ°(H+)=349.6 S cm2 mol-1 andλ°(HCOO-) = 54.6 S cm2 mol-1
Answer: 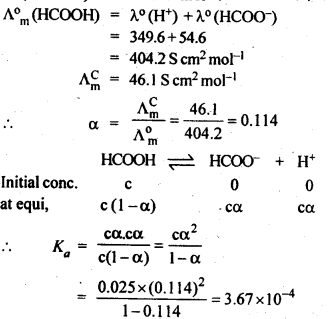
Question 10. If a current of 0.5 ampere flows through a metallic wire for 2 hours, then how many electrons flow through the wire?
Answer: Q = I × t = (0.5 ampere) × (2 × 60 × 60) = 3600 C. A flow of 1 F means 96500 C is equivalent to flow of 1 mole of electrons or 6.022 × 1023 electrons. ∴ 3600 C is equivalent to flow of electrons = (6.022 × 1023) × 3600 / 96500 = 2.246 × 1022 electrons.
Question 11. Suggest a list of metals that are extracted electrolytically.
Answer : Sodium, calcium, magnesium and aluminium.
Question 12. Consider the reaction: Cr2O72-+ 14H+ + 6e– → 2Cr3+ + 7H2O What is the quantity of electricity in coulombs needed to reduce 1 mol of Cr2O72- ?
Answer: According to the given reaction, it is clear that 1 mol of Cr2O72- ions gains 6 mole of electrons that means this process requires 6F electricty = 6 × 96500 C of electricty for reduction to Cr3+.
Question 13. Write the chemistry of recharging the lead storage battery highlighting all the materials that are involved during recharging.
Answer: A lead storage battery consists of anode of lead, cathode of a grid of lead packed with lead dioxide (PbO2) and 33% solution of Sulphuric acid as electrolyte. When the battery is in use ,the following reactions take place: 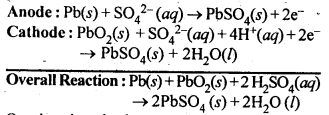
On charging the battery, the reverse reaction takes place that means PbSO4 deposited on the electrodes is converted back into Pb and PbO2 and H2SO4 is regenerated.
Question 14. Suggest two materials other than hydrogen that can be used as fuels in fuel cells.
Answer: Methane and methanol.
Question 15. Explain how rusting of is envisaged as setting up of an electrochemical cell.
Answer: The water layer present on the surface of iron (especially in the rainy season) dissolves acidic oxides of the air like CO2, SO2 etc. to form acids which dissociate to give H+ ions in the following way: 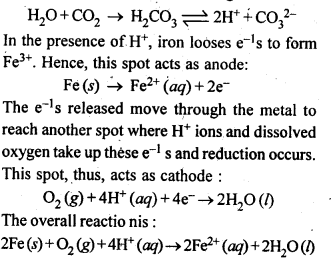
Thus an electrochemical cell is set up on the surface. Ferrous ions are further oxidised by the atmospheric oxygen to ferric ions which combine with water molecules to form hydrated ferric oxide, Fe2O3. XH2O, that is rust.
Answers of Exercise Questions of Ncert Solution of Electrochemistry
Question 1. Arrange the following metals in the order in which they displace each other from the solution of their salts: Al, Cu, Fe, Mg and Zn.
Answer: Mg, Al, Zn, Fe, Cu.
Question 2. Given the standard electrode potentials, K+/K = -2. 93 V, Ag+/Ag = 0.80 V, Hg2+/Hg = 0.79V, Mg2+/Mg = -2.37V, Cr3+/Cr = 0.74V.
Arrange these metals in their increasing order of reducing power.
Answer: As we know that higher the oxidation potential, more easily it is oxidised and hence greater is the reducing power. Thus, increasing order of reducing power will be Ag < Hg < Cr < Mg <K.
Question 3. Depict the galvanic cell in which the reaction
Zn(s) + 2Ag+(aq) —-> 7M2+(aq) + 2Ag (s) takes place. Further show:
(i) Which of the electrode is negatively charged?
(ii) The carriers of the current in the cell.
(iii) Individual reaction at each electrode.
Answer: The set up of the galvanic cell will be similar as to shown below: 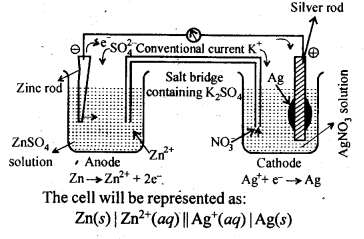
(i) Anode that means zinc electrode will be negatively charged. (ii) The current will flow from silver to copper in the external circuit. (iii) At anode: Zn(s) → Zn2+ (aq) + 2e and at cathode: Ag+ (aq) + e → Ag (s).
Question 4. Calculate the standard cell potentials of the galvanic cells in which the following reactions take place. 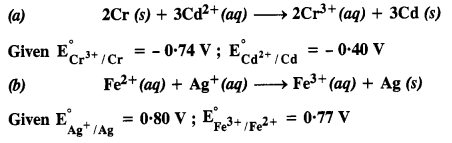
Also calculate ΔrGo and equilibrium constant of the reactions.
Answer: (i) Eocell = Eocathode – Eoanode = – 0.40 V – (– 0.74 V) = + 0.34 V. ΔrGo = – nFEocell = – 6 mol × 96500 C mol–1 × 0.34 V = – 196860 CV mol—1 = – 196860 J mol-1 = – 196.86 KJ mol-1 and – ΔrGo = 2.303 RT log K or 196860 = 2.303 × 8.314 × 298 log K or log K = 34.5014 or K = Antilog 34.5014 = 3.193 × 1034 .
(ii) Eocell = + 0.80 – 0.77 V = + 0.03 V. ΔrGo = – nFEocell = – 1 mol × 96500 C mol–1 × 0.03 V = – 2895 CV mol—1 = – 2895 J mol-1 = – 2.895 KJ mol-1 and – ΔrGo = 2.303 RT log K or – 2895 = – 2.303 × 8.314 × 298 log K or log K = 0.5074 or K = Antilog 0.5074 = 3.22.
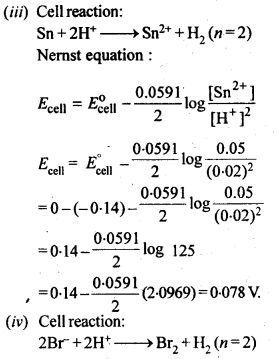
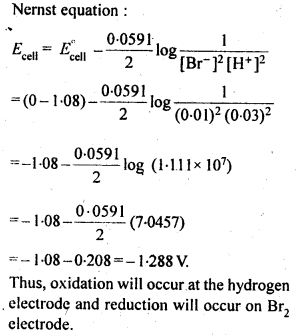
Question 5. Write the Nernst equation and emf of the following cells at 298 K: (i) Mg (s) | Mg2+ (0.001M) || Cu2+ (0.0001M) | Cu (s). (ii) Fe (s) | Fe2+ (0.001M) || H+ (1M) | H2 (g) (1bar)| Pt (s) (iii) Sn (s) |Sn2+ (0.050M) || H+ (0.020M) | H2 (g) (1bar)| Pt (s) (iv) Pt (s) | Br2 (l) | Br– (0.010 M)|| H+ (0.030M) | H2 (g) (1bar)| Pt (s)
Given: Eo Mg2+/Mg = – 2.37 V, Eo Cu2+/ Cu = + 0.34 V, Eo Fe2+/Fe = – 0.44 V, Eo Sn2+/Sn = – 0.14 V, Eo 1/2Br2/Br– = + 1.08 V.
Answer: 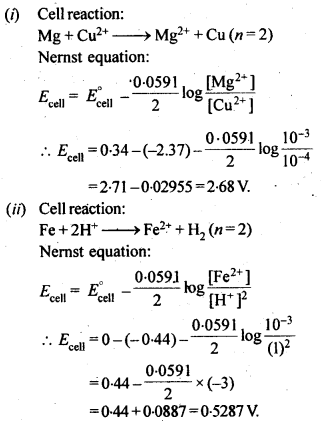
Question 6. In the button cells widely used in watches and other devices the following reaction takes place: 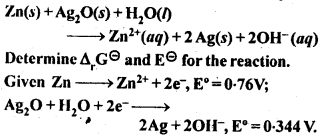
Answer: 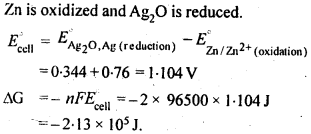
Question 7. Define conductivity and molar conductivity for solution of an electrolyte. Discuss their variation with concentration.
Answer: Conductivity. The reciprocal of resistivity is called specific or simple conductivity. It is denoted by κ (kappa). And κ = G × l/a where G = conductance, l = length of wire and a = area of cross section. Unit of κ is ohm-1 cm-1 or S cm-1..
Molar conductivity . The conductance of all the ions produced from one mole of the electrolyte dissolved in V cm3 of the solution when the electrodes are one cm apart and the area of the electrodes is so large that the whole of the solution is contained between them is called molar conductivity. It is denoted by ∧mc. And ∧mc = κv × V or ∧mc = kv × 1000 / molarity. Its unit is S cm2 mol-1.
Variation of Molar conductivity with concentration: Variation of molar conductivity with concentration depends on the type of electrolytes. In case of strong electrolyte, this effect can be understood by Debye Huckle- Onsager equation as ∧cm = ∧om – √c. The graph plotted ∧m versus √c, it was found that there is a small increase in conductance with dilution.
At higher concentration, the greater inter Ionic attractions retard the motion of ions and therefore, the conductance falls with increasing concentration. On the contrary, the conductance increases with dilution and approaches a maximum limiting value at infinite dilution and at this stage it is called limiting molar conductivity. It is denoted by ∧om or ∧∞m . 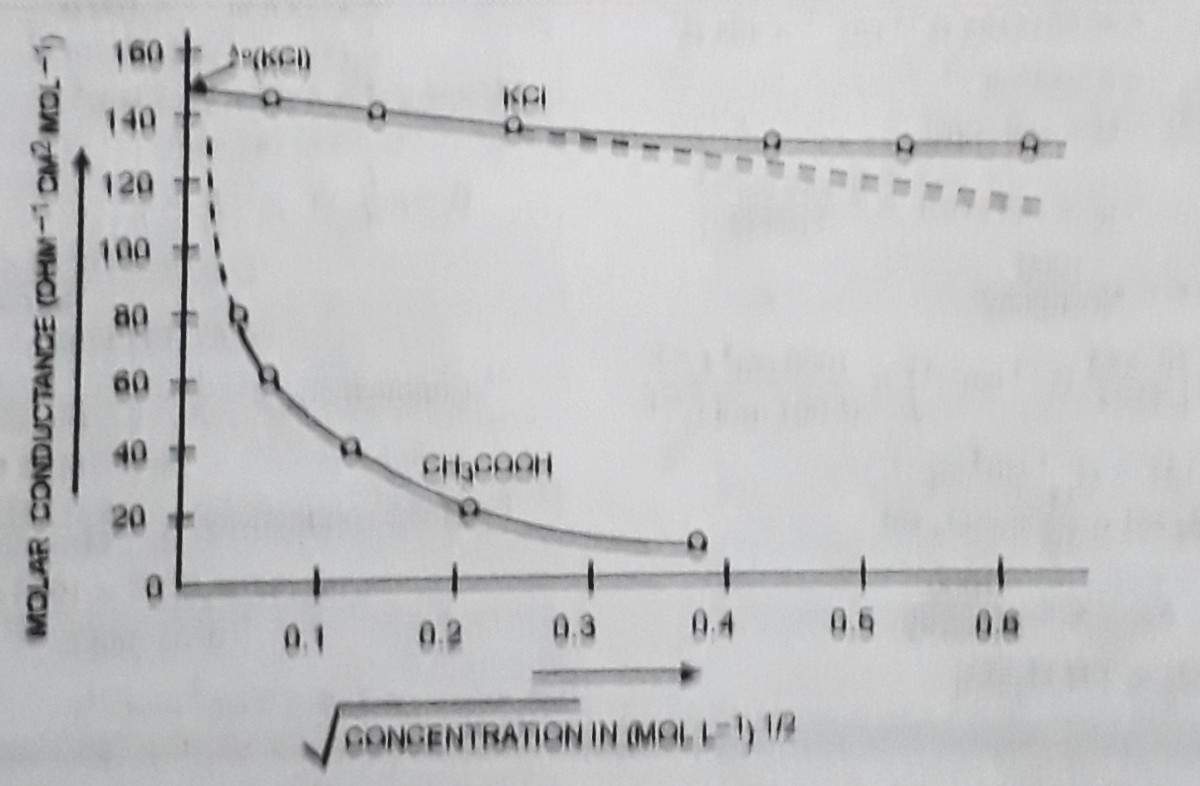
In case of weak electrolyte. The molar conductivity of this electrolyte is lower at high concentrations with respect to strong electrolyte and its gets unlimited increase at infinite dilution.
Question 8. The conductivity of 0.20 M solution of KCl at 298 K is 0.0248 S cm-1. Calculate its molar conductivity.
Answer: ∧mc = kv × 1000 / molarity = (0.0248 S cm-1 × 1000 cm3 L–1) / 0.20 mol L-1 = 124 S cm2 mol-1.
Question 9. The resistance of a conductivity cell containing 0.001 M KCl solution at 298 K is 1500 ohm. What is the cell constant if conductivity of 0.001 M KCl solution at 298 K is 0.146 × 10–3 S cm-1?
Answer: Cell constant = conductivity/ conductance = conductivity × resistance = 0.146 × 10–3 S cm-1 × 1500 ohm = 0.219 cm-1
Question 10. The conductivity of NaCl at 298 K has been determined at different concentrations and the results are given below: 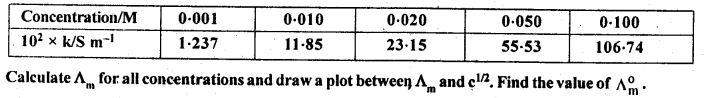
Answer: Here 1S cm-1 = 100 S m-1 and 1 S cm-1/ 100 S m-1 = 1 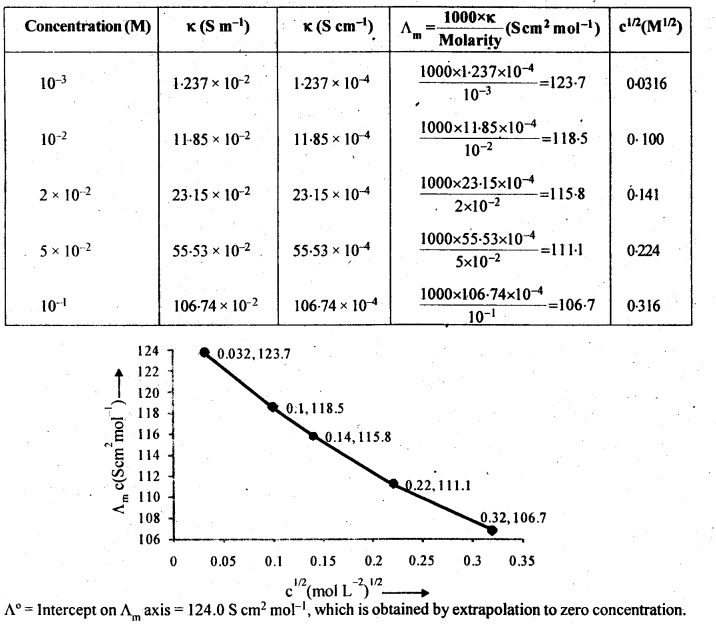
Question 11. Conductivity of 0.00241 M acetic acid is 7.896 x 10-5 S cm-1. Calculate its molar conductivity. If Λm0, for acetic acid is 390.5 S cm2 mol-1, what is its dissociation constant?
Answer: 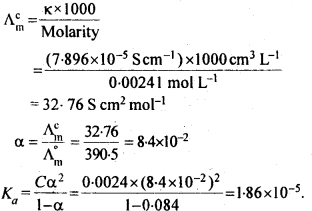
Question 12. How much charge is required for the following reductions:
(i) 1 mol of Al3+ to Al?
(ii) 1 mol of Cu2+ to Cu ?
(iii) 1 mol of Mn04- to Mn2+?
Answer: (i) The electrode reaction can be written as: Al3+ + 3e → Al ∴ Quantity of charge required for reduction of 1 mol of Al3+ = 3F = 3 × 96500 C = 289500 C. (ii) The electrode reaction can be written as: Cu2+ + 2e → Cu ∴ Quantity of charge required for reduction of 1 mol of Cu2+ = 2F = 2 × 96500 C = 193000 C. (iii) The electrode reaction can be written as: MnO4– → Mn2+ or Mn7+ + 5e → Mn2+ ∴ Quantity of charge required for reduction of 1 mol of MnO4– = 5F = 5 × 96500 C = 482500 C.
Question 13. How much electricity in terms of Faraday is required to produce :
(i) 20·0 g of Ca from molten CaCl2
(ii) 40·0 g of Al from molten Al2O3 ?
Answer: (i) Ca2+ + 2e → Ca. That means 1 mol of Ca or 40 g of Ca require electricity = 2F. ∴ 20 g of Ca will need electricity = 1F. (ii) Al3+ + 3e → Al. That means 1 mol of Al or 27 g of Al require electricity = 3F. ∴ 40 g of Al will need electricity = (3/27) × 40 = 4.44 F.
Question 14. How much electricity is required in coulomb for the oxidation of (i) 1 mol of H2O to 02 (ii) 1 mol of FeO to Fe203
Answer: (i) The electrode reaction for 1 mol of H2O is: H2O → H2 + 1/2 O2 or O2– → 1/2 O2 + 2e ∴ Quantity of electricity required = 2F = 2 × 96500 C = 193000 C. (ii) The electrode reaction for 1 mol of FeO to convert into Fe2O3 is: FeO → 1/2 Fe2O3 or Fe2+ → Fe3+ + e ∴ Quantity of electricity required = 1F = 96500 C.
Question 15. A solution of Ni(N03)2 is electrolyzed between platinum electrodes using a current of 5 amperes for 20 minutes. What mass of Ni is deposited at the cathode?
Answer: Quantity of electricity passed = (5A) × (20 × 60 s) = 6000 C. And Ni2+ + 2e → Ni thus quantity of electricity = 2F = 2 × 96500 C of electricty deposits 1 mol Ni or 58.7 g Ni. ∴ 6000 C of electricty will deposit Ni = (58.7 × 6000) / (2 × 96500) = 1.825 g.
Question 16. Three electrolytic cells A, B, C containing solutions of ZnS04, AgNO3 and CuS04, respectively are connected in series. A steady current of 1.5 amperes was passed through them until 45 g of silver deposited at the cathode of call B. How long did the current flow? What mass of copper and zinc were deposited?
Answer: Ag+ + e → Ag . This reaction implies that 108 g of Ag are deposited by 1F = 96500 C ∴ 1.45 g of Ag will be deposited by (96500 × 1.45)/108 = 1295.6 C. As we know that Q = I × t or t = Q/I = 1295.6/1.50 = 863.7 s = 14 min.24 sec.
Cu2+ + 2e → Cu that means 2 × 96500 C deposit Cu = 63.5 g. ∴ 1295.6 C will deposit Cu = (63.5 × 1295.6) / (2 × 96500) = 0.426. Again Zn2+ + 2e → Zn ∴ Zn deposited = (65.3 × 1295.6) / (2 × 96500) = 0.438 g.
Question 17. Using the standard electrode potentials given in the table, predict if the reaction between the following is feasible.
(a) Fe3+(aq) and I–(aq)
(b) Ag+(aq) and Cu(s)
(c) Fe3+(aq) and Br–(aq)
(d) Ag(s) and Fe3+(aq)
(e) Br2(aq) and Fe2+(aq).
Given standard electrode potentials: Eo 1/2 I2, I– = 0.541, EoCu2+, Cu = 0.34 V, Eo1/2Br2, Br– = 1.09 V, EoAg+,Ag = + 0.80 V, EoFe3+, Fe2+ = + 0.77 V
Answer: A Redox reaction is feasible only when the standard cell potential of this cell is zero. Keeping this concept in mind we shall predict that the given cell reaction is feasible or not. 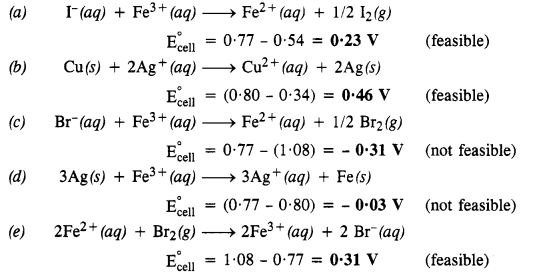
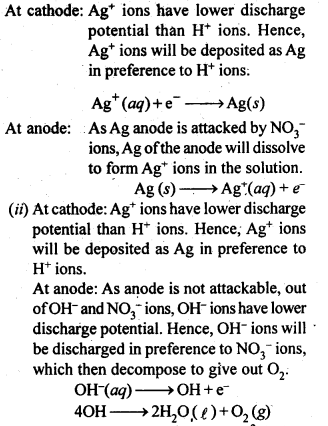
Question 18. Predict the products of electrolysis in each of the following.
(i) An aqueous solution of AgNO3 with silver electrodes.
(ii) An aqueous solution of AgNO3 with platinum electrodes.
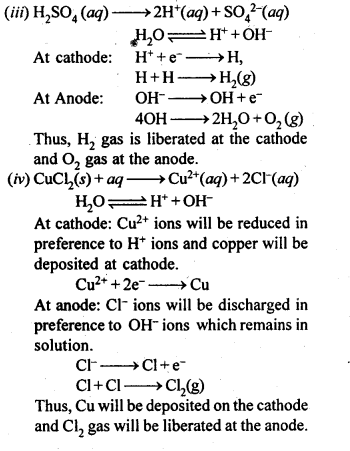
(iii) A dilute solution of H2S04 with platinum electrodes.
(iv) An aqueous solution of CuCl2 with platinum electrodes.
Answer: 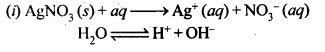
Conclusion of Ncert Solution of Electrochemistry
You all are welcome to visit this site. We have solved all the questions to be asked either in intext or exercise of Ncert Solution of Electrochemistry For Class 12th. We hope that you all must have liked this solution notes. If you all have any doubts or suggestions, please write inform us writing in comment box. We shall again request you to share this notes among your friends and favourites.

