Ncert Solution of Ionic Equilibrium is for class 11th. This is the part of Equilibrium chapter 6. We have divided this chapter into two parts as chemical equilibrium and Ionic equilibrium. This was done so that students can not bore during the the study or solution of this chapter. We have included all the questions in solution which have been given in the exercise of Ncert book. There are all over 39 questions from Ionic equilibrium.
We have solved all the 39 questions of Ionic equilibrium. These questions are started from 35 and ended at 73. In Ncert Solution of Ionic Equilibrium, All the questions have been solved in simple and easy methods. About all the concepts have been taken into consideration so that a complete revision can be done of this chapter. The topics of this chapter are very important for the another part of this chemistry. Students must read this chapter many times and solve the answer of the questions of exercise given in ncert book.
This is chapter from which numbers of questions are asked in NEET, Jee(mains) and IIT advance. Some other competitive exams also have value in this chapter. Students should have a good knowledge of the topics given in this chapter. Ncert Solution of Ionic Equilibrium will prove the mile stone in preparation of this chapter. We have made available free pdf of Ncert Solution of Ionic Equilibrium that can download below: —
Ncert Solution of Ionic Equilibrium Overview
There are a lot of things to learn in this chapter.
- We know what are called acids, bases and salts.
- What learn to classify as acids or bases according to Arrhenius, Bronsted-Lowery and Lewis concepts.
- To classify acids and bases as weak or strong in terms of their ionisation constant.
- To explain the dependence of degree of ionization on concentration of the electrolyte and that of the common ion
- To describe the pH scale for representing hydrogen ion concentration
- To explain ionization of water and its dual role as acid and base.
- To appreciate use of Buffer solutions.
- To calculate the solubility product of sparingly soluble salt.
Question and Solution of Ionic equilibrium :
Q.No. 35 What is meant by the conjugate acid-base pair ? Find the conjugate acid/ base for the following species: HNO2, CN–, HClO4, F–, OH–, CO32–, and S2–
Ans:- An acid-base pair which differ by a proton (H+) is called conjugate acid-base pair. NO2–, HCN, ClO4–, HF, H2O(acid) or O2–, HCO3– and HS–.
Q.No.36 Which of the following are Lewis acids? H2O, BF3, H+, NH4+. (Ncert Solution of Ionic Equilibrium)
Ans:- BF3, H+, and NH4+ are Lewis acids.
Q.No.37 What will be the conjugate bases for the Bronsted acids: HF, H2SO4, and HCO3– .
Ans:- We know that Conjugate acid ⇌ Conjugate base + H+ or Conjugate base ⇌ Conjugate acid – H+ ∴ Conjugate bases for the given acids will be F–, HSO4 –, CO32–.
Q.No.38 Write the Conjugate acids for the following Bronsted bases: NH2–, NH3 and HCOO–. (Ncert Solution of Ionic Equilibrium)
Ans:- NH3, NH4–, HCOOH .
Q.No.39 The species: H2O, HCO3–, HSO4 –, and NH3 can act both as Bronsted acids and bases. For each case give the corresponding conjugate acid and base.
Ans:- Conjugate acid: H3O+, H2CO3, H2SO4, NH4+. Conjugate bases: OH—, CO32–, SO42–, NH2–.
Q.No. 40 Classify the following species into Lewis acids and Lewis bases and show how these act as Lewis acid/base: (a) OH– (b) F– (c) H+ (d) BCl3 .
Ans:- (a) OH– can donate electron pair, Hence, it is a Lewis base. (b) F– can also donate electron pair. Hence, it is a Lewis base. (C) H+ can accept electron pair. Hence it is a Lewis acid. (d) BCl3 is deficient in electrons. Hence, it can accept electron pair and is, therefore, a Lewis acid.
Q.No.41 The concentration of hydrogen ion in a sample of soft drink is 3.8 × 10–3 M. What is its pH ? (Ncert Solution of Ionic Equilibrium)
Ans:- pH = – log [H+] = – log (3.8 ×10–3) = – log 3.8 + 3 = 3 – 0.5798 = 2.402 = 2.42.
Q.No.42 The pH of a sample of vinegar is 3.76. Calculate the concentration of hydrogen ion in it. (Ncert Solution of Ionic Equilibrium)
Ans:- pH = – log [H+] or log [H+] = – pH = – 3.76 = 4¯.24 ∴ [H+] = Antilog ‾4.24 = 1.738 × 10–4 M = 1.74 × 10–4 M.
Q.No.43. The ionization constant for HF, HCOOH and HCN at 298 K is 6.8 × 10–4, 1.8 × 10–4 and 4.8 × 10–9 respectively. Calculate the ionization constant of the corresponding conjugate base.
Ans:- For F–, Kb = Kw/Ka = 10–14/ (6.8 × 10–4 = 1.47 × 10–11 = 1.5 × 10–11
For HCOOH, Kb = 10–14/ (1.8 × 10–4 = 1.47 × 10–11 = 1.5 × 10–11 and For HCN, Kb = 10–14/ (4.8 × 10–9 )= 2.08 × 10–6 .
Q.No 44. The ionization constant of Phenol is 1.8 × 10–10. What is the concentration of Phenol in 0.05 M solution of Phenol ? What will be its degree of ionization if the solution is also 0.01 M in sodium phenate ? (Ncert Solution of Ionic Equilibrium)
Ans:- 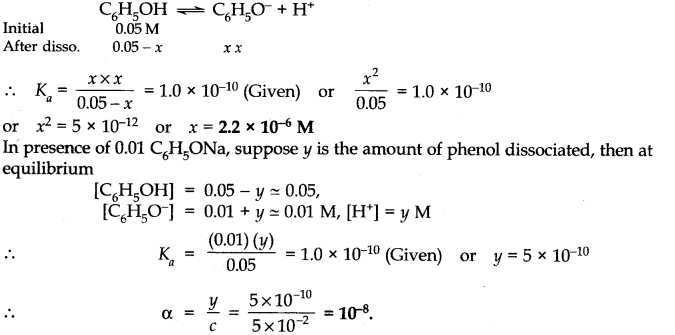
Q.No. 45. The first ionization constant of H2S is 9.1 × 10–8. Calculate the concentration of HS– ions in its 0.1 M solution and how will this concentration be affected if the solution is 0.1 M in HCl also ? If the second ionization constant of H2S is 1.2 × 10–13, Calculate the concentration of S2– under both conditions.
Ans:- 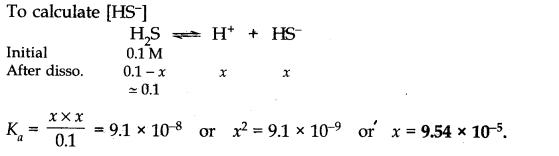
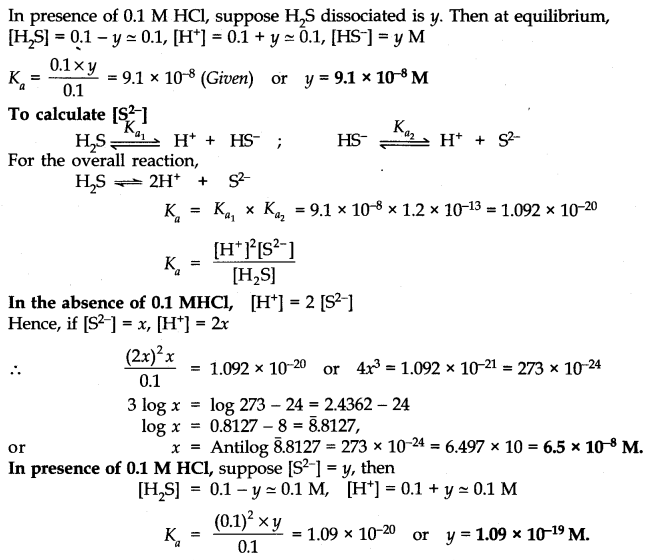
Q.No. 46. The ionization constant of acetic acid is 1.74 × 10–5. Calculate the degree of dissociation of acetic acid in its 0.05 M solution. Calculate the concentration of acetic ions in the solution and its pH. (Ncert Solution of Ionic Equilibrium)
Ans:- 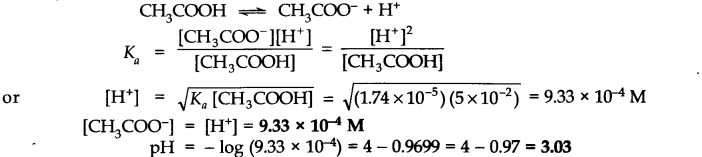
Q.No. 47. It has been found that the pH of a 0.01M solution of an organic acid is 4.15. Calculate the concentration of the anion, the ionization constant of the acid and its pKa. (Ncert Solution of Ionic Equilibrium)
Ans:- 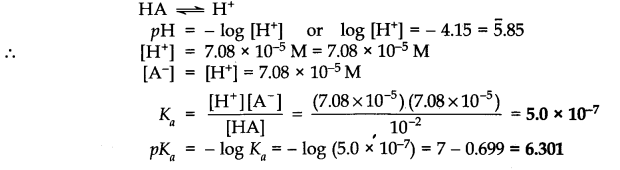
Q.No. 48. Assuming complete dissociation, calculate the pH of the following solutions: (a) 0.003 M HCl (b) 0.005 M NaOH (c) 0.002 M HBr (d) 0.002 M KOH.
Ans:- 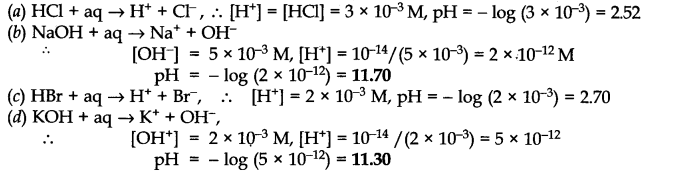
Q.No. 49. Calculate the pH of the following reactions: (a) 2 g of TiOH dissolved in water to give 2 litre of the solution. (b) 0.3 g of Ca(OH)2 dissolved in water to give 500 ml of the solution. (c) 0.3 g of NaOH dissolved in water to give 200 ml of the solution. (d) 1 ml of 13.6 M HCl is diluted with water to give 1 Litre of the solution.
Ans:- 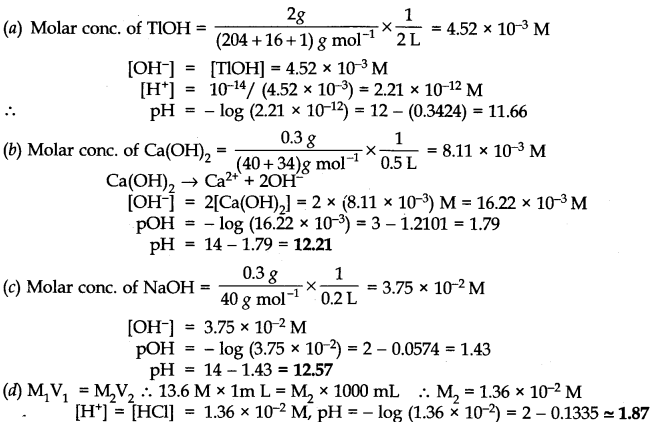
Q.No. 50. The degree of ionization of a 0.1 M bromoacetic acid solution is 0.132. Calculate the pH of the solution and the pKa of bromoacetic acid.
Ans:- 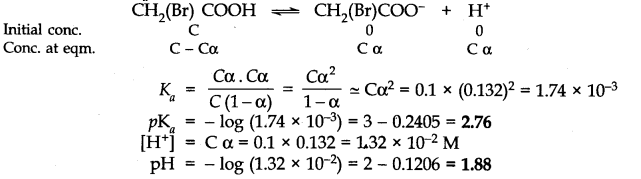
Q.No. 51. The pH of 0.005 M codeine (C18H21NO3) solution is 9.95. Calculate the ionization constant and pKb.
Ans:- 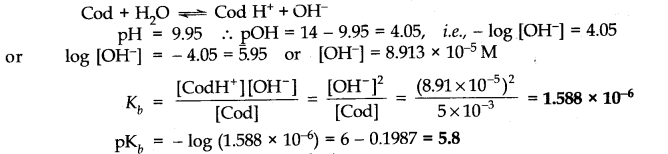
Q.No. 52. The pH of 0.001 M Aniline solution ? The ionization constant of Aniline is 4.27 × 10–10. Calculate degree of ionization of Aniline in the solution. Also calculate the ionization constant of the conjugate acid of Aniline.
Ans:- 
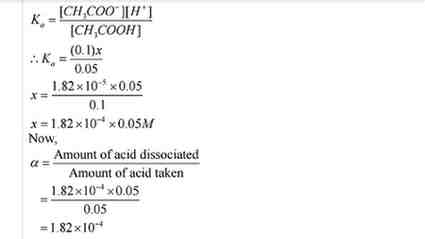
Q.No. 53. Calculate the degree of ionization of 0.05 M of acetic acid if its pKa value is 4.74. How is the degree of dissociation affected when its solution contains (a) 0.01 M (b) 0.1 M HCl ?
Ans:- 
Q.No. 54. The ionization constant of dimethylamine is 5.4 × 10–4. Calculate the degree of ionization in its 0.02 M solution. What percentage of dimethylamine is ionized if the solution is also 0.1 M NaOH ? (Ncert Solution of Ionic Equilibrium)
Ans:- 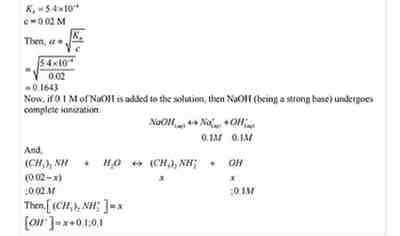

Q.No. 55. Calculate the hydrogen ion concentration in the following biological fluids whose pH given below:
(a) Human muscle-fluid, 6.83 (b) Human stomach fluid, 1.2 (c) Human blood, 7.38 (d) Human saliva, 6.4.
Ans:- (a) log [H+] = – pH = – 6.83 = 7‾.17 ∴ [H+] = Antilog 7‾.17 = 1.479 × 10–7 M.
(b) log [H+] = – pH = – 1.2 = 2‾.8 ∴ [H+] = Antilog 2‾.8 = 6.31 × 10–2 M.
(c) log [H+] = – pH = – 7.38 = 8‾.62 ∴ [H+] = Antilog 8‾.62 = 4.169 × 10–8 M.
(d) log [H+] = – pH = – 6.4 = 7‾.60 ∴ [H+] = Antilog 7‾.60 = 3.981 × 10–7 M.
Q.No.56. The pH of milk, black coffee, tomato juice, lemon juice and egg white are 6.8, 5.0, 4.2, 2.2 and 7.8 respectively. Calculate corresponding hydrogen ion concentration in each. (Ncert Solution of Ionic Equilibrium)
Ans: For milk, log [H+] = – pH = – 6.8 = 7‾.20 ∴ [H+] = Antilog 7‾.20 = 1.5 × 10–7 M.
For black coffee, log [H+] = – pH = – 5.0 ∴ [H+] = 10–5 M
For tomato juice: log [H+] = – pH = – 4.2 = 5‾.80 ∴ [H+] = Antilog 5‾.80 = 6.31 × 10–5 M.
For lemon juice: log [H+] = – pH = – 2.2 = 3‾. 8 ∴ [H+] = Antilog 3‾.8 = 6.31 × 10–3 M.
For egg white: log [H+] = – pH = – 7.8 = 8‾.20 ∴ [H+] = Antilog 8‾.20 = 1.58 × 10–8 M.
Q.No.57. If 0.561 g KOH is dissolved in water to give 200 mL of solution at 298 K. Calculate the concentration of potassium, hydrogen and hydroxyl ions. What is its pH ? (Ncert Solution of Ionic Equilibrium)
Ans: [KOH] = (0.561/56) × (1000/200) = 0.050 M. As KOH → K+ + OH–, ∴ [K+] = [OH–] = 0.050 M. Now, [H+] = Kw /[OH–] = 10–14 /0.05 = 10–14 / 5 × 10–2 = 2.0 × 10–13 M. and pH = – log [H+] = – log(2.0 × 10–13 ) = 13 – 0.301 = 12.699.
Q.No. 58. The solubility of Sr(OH)2 at 298 K is 19.23 g/L of solution. Calculate the concentration of strontium and hydroxyl ions and pH of the solution. (Atomic mass of Sr = 87.6)
Ans:- Molar mass of Sr(OH)2 = 87.6 +34 = 121.6 gmol-1 and solubility of Sr(OH)2 in molesL–1 = 19.23 g L–1 / 121.6 g mol-1 = 0.1581 M.
Assuming complete dissociation, Sr(OH)2 → Sr2+ + 2OH– ∴ [Sr2+] = 0.1581 M and [OH–] = 2 × 0.1581 = 0.3162 M. And pOH = – log 0.3162 = 0.5 ∴ pH = 14 – 0.5 = 13.5.
Q.No. 59. The ionization constant of propanoic acid is 1.32 × 10–5. Calculate the degree of ionization if its solution is 0.05 M. What will be its degree of ionization if the solution is 0.01 M in HCl also ? (Ncert Solution of Ionic Equilibrium)
Ans:- Let the degree of propanoic acid be α and then, representing propanoic acid as HA, we have: 
Q.No. 60. The pH of 0.1 M solution of cyanic acid (HCNO) is 2.34. Calculate the ionization constant of the acid and the degree of ionization in the solution.
Ans:- 
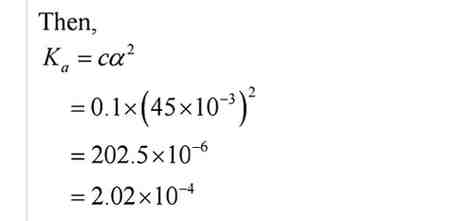
Q.No. 61. The ionization constant of nitrous acid is 4.5 × 10–4. Calculate the pH of 0.04 M sodium nitrite solution and also its degree of hydrolysis.
Ans:- Sodium nitrite is a salt of weak acid and strong base. And 
Q.No. 62. A 0.02 M solution of pyridinium hydrochloride has pH = 3.44. Calculate the ionization constant of pyridine. (Ncert Solution of Ionic Equilibrium)
Ans:- 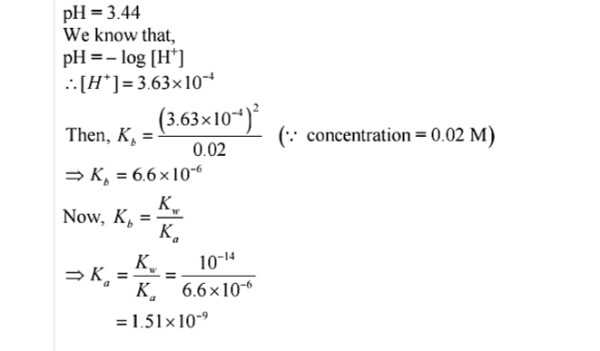
Q.No. 63. Predict the acidic, basic or neutral nature of the solutions of the following salts: NaCl, KBr, NaCN, NH4NO3, NaNO2, KF.
Ans:- NaCN, NaNO2 and KF solutions are basic, as they are salts of strong base and weak acid. NaCl and KBr solutions are neutral, as they are salts of strong acid and strong base. While NH4NO3 is acidic, as it is a salt of strong acid and weak base.
Q.No. 64. The ionization constant of chloroacetic acid is 1.35 × 10–3. What will be the pH of 0.1M acid and its 0.1 M sodium salt solution ? (Ncert Solution of Ionic Equilibrium)
Ans:- 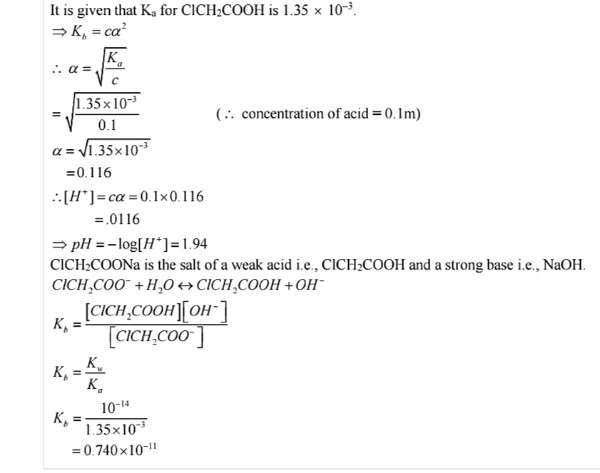
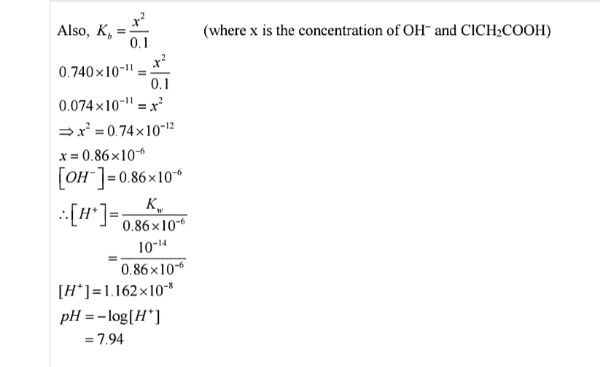
Q.No. 65. The Ionic product of water at 310 K is 2.7 × 10–14. What is the pH of neutral water at this temperature ?
Ans:- [H+] = √Kw = √2.7 × 10–14 = 1.643 × 10–7 M. Or pH = — log [H+] = – log (1.643 × 10–7) = 7 – 0.2156 = 6.78
Q.No 66. Calculate the pH of the resultant mixtures. (a) 10 mL of 0.2 M Ca(OH)2 + 25 mL of 0.1 M HCl (b) 10 mL of 0.01 M H2SO4 + 10 mL of 0.01 M Ca(OH)2 (c) 10 mL of 0.1 M H2SO4 + 10 ml of 0.1 M KOH. (Ncert Solution of Ionic Equilibrium)
Ans:- (a) 10 mL of 0.2 M Ca(OH)2 = 10 × 0.2 millimoles = 2 millimoles of Ca(OH)2 and 25 mL of 0.1 M HCl = 25 × 0.1 millimoles = 2.5 millimoles of HCl. Reaction between them takes place in the following way.
Ca(OH)2 + 2 HCl → CaCl2 + 2 H2O, In this reaction 1 millimoles of Ca(OH)2 reacts with 2 millimoles of HCl.
∴ 1.25 millimoles of Ca(OH)2 will react with 2.5 millimoles of HCl. Hence, Ca(OH)2 left = 2 – 1.25 = 0.75 millimoles as HCl is the limiting reactant. Now total volume of the solution = 10 + 25 mL = 35 mL.∴ molarity of Ca(OH)2 in the mixture solution = 0.75/35 M = 0.0234 M.
∴ [OH–] = 2 × 0.0214 M = 0.0428 M = 4.28 × 10–2. Now, pOH = – log(4.28 × 10–2) = 2 – 0.6314 = 1.37 ∴ pH = 14 – 1.37 = 12.63
(b) 10 mL of 0.01 M H2SO4 = 10 × 0.01 millimoles = 0.1 millimoles of H2SO4 and 10 mL of 0.01 M Ca(OH)2 = 10 × 0.01 millimoles = 0.1 millimoles of Ca(OH)2. As Ca(OH)2 + H2SO4 → CaSO4 + 2H2O that means 1 mole of Ca(OH)2 reacts with 1 mole of H2SO4 ∴ 0.1 millimole of Ca(OH)2 will react completely with 0.1 millimole of H2SO4. Hence, solution will be neutral with pH = 7.0
(c) 10 mL of 0.1M H2SO4 = 1 millimole, 10 mL of 0.1M KOH = 1 millimole. The reaction between them as below:
2 KOH + H2SO4 → K2SO4 + 2H2O means 1 millimole of KOH will react with 0.5 millimole of H2SO4 ∴ H2SO4 left = 1 – 0.5 = 0.5 millimole. Now Volume of reaction mixture = 15 + 10 = 35 mL. ∴ molarity of H2SO4 in the mixture solution = 0.5/20 = 2.5 × 10–2 M. Now [H+] = 2 × 2.5 × 10–2 = 5 × 10–2 ∴ pH = – log (5 × 10–2) = 2 – 0.699 = 1.3
Q.No. 67. Determine the solubilities of Silver dichromate, barium chromate, ferric hydroxide, lead chloride and mercurous iodide at 298 K. Given Ksp values: Ag2CrO4 = 1.1 × 10–12, BaCrO4 = 1.2 × 10–10, Fe(OH)3 = 1.0 × 10–38, PbCl2 = 1.6 × 10–5, Hg2I2 = 4.5 × 10–29. Determine also the molarities of individual ions. (Ncert Solution of Ionic Equilibrium)
Ans:- 

Q.No. 68. The solubility of product constants of Ag2CrO4 and AgBr are 1.1 × 10–12 and 5.0 × 10–13 respectively. Calculate the ratio of molarities of their saturated solutions.
Ans:- Let ‘s’ be the solubility of Ag2CrO4 then Ag2CrO4 → 2Ag+ + CrO42– Now 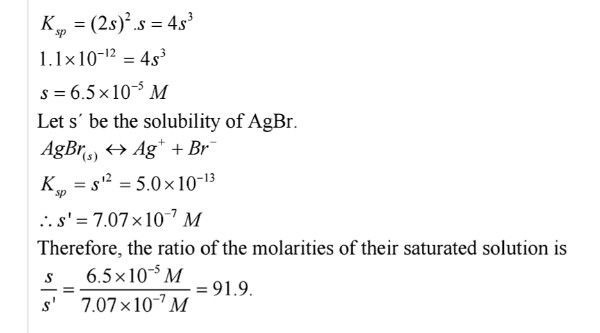
Q.No . 69. Equal volumes of 0.002 M solutions of sodium iodate and copper chlorate are mixed together. Will it lead to precipitation of Copper iodate ? For Copper iodate, Ksp = 7.4 × 10–8.
Ans: 2NaIO3 + CuCrO4 → Na2CrO4 + Cu(IO3)2 . After mixing, [NaIO3] = [IO3–] = 2 × 10–3 /2 = 10–3 M and [CuCrO4] = [Cu2+] = 2 × 10–3/2 = 10–3 M. Now Ionic product of Cu[IO3]2 = [Cu2+] [IO3–]2 = (10–3) (10–3)2 = 10–9. As Ionic product is less than Ksp, no precipitation will occur.
Q.No. 70. The ionization constant of benzoic acid is 6.46 × 10–5 and Ksp for silver benzoate is 2.5 × 10–13. How many times is silver benzoate more soluble in a buffer of pH 3.19 compared to its solubility in pure water ? (Ncert Solution of Ionic Equilibrium)
Ans:- 
Q.No. 71. What is the maximum concentration of equimolar solutions of ferrous sulphate and sodium sulphide so that when mixed in equal volumes. There is no precipitation of iron sulphide? For iron sulphide, Ksp = 6.3 × 10–18.
Ans:- Suppose the concentration of each of FeSO4 and Na2S is x mol L-1. Then after mixing equal volume, [FeSO4] = [Na2S] = x/2 M, i.e., [Fe2+] = [S2–] = x/2 M. Now, Ksp for FeS = [ Fe2+][S2–] , i.e., 6.3 × 10–18 = x/2 × x/2 or x2 = 25.2 × 10–18 ∴ x = 5.02 × 10–9 M.
Q.No. 72. What is the minimum Volume of water required to dissolve 1 g of calcium sulphate at 298 K. For calcium sulphate, Ksp = 9.1 × 10–6. (Ncert Solution of Ionic Equilibrium)
Ans:- CaSO4 (s) ⇌ Ca2+ (aq) + SO42- (aq) If ‘s’ is the solubility of CaSO4 in molesL–1, then Ksp = [Ca2+] × [SO42-] = s2 or s = √Ksp = √(9.1 × 10–6 = 3.02 × 10–3 molL–1 = 3.02 × 10–3 × 136 gL–1 = 0.411 gL–1 (Molar mass of CaSO4 = 136 gmol-1)
Thus, for dissolving 0.411 g, water required = 1 L, ∴ For dissolving 1 g, water required = 1/0.411 L = 2.43 L.
Q.No.73. The concentration of sulphide ion in 0.1 M HCl solution saturated with hydrogen sulphide is 1.0 × 10–19 M. If 10 mL of this solution is added to 5 mL of 0.04 M solution of FeSO4, MnCl2, ZnCl2 and CaCl2, in which solution precipitation take place ? Given Ksp for FeS = 6.3 × 10–18, MnS = 2.5 × 10–13, ZnS = 1.6 × 10–24 and CdS = 8.0 × 10–27. (Ncert Solution of Ionic Equilibrium)
Ans:- precipitation will take place in the solution for which Ionic product is greater than solubility product. As 10 mL solution containing S2– ion is mixed with 5 mL of metal salt solution, after mixing [S2–] = (1.0 × 10–19) × 10/15 = 6.67 × 10–20, [Fe2+] = [Mn2+] = [Zn2+] = [Cd2+] = 5/15 × 0.04 = 1.33 × 10–2 M.
∴ Ionic product for each of these will be = [M2+][S2–] = (1.33 × 10–2) × (6.67 × 10–20) = 8.87 × 10–22. As this is greater than the solubility product of ZnS and CdS, therefore, ZnCl2 and CdCl2 solution will be precipitated.

