Ncert Solution of Organic Chemistry: Here, we shall learn to solve the questions of some basic Principles and Techniques of organic chemistry. This is the chapter of class 11th. This is the basic chapter of organic chemistry and this is the chapter 8 according to this revised syllabus of Ncert patterns. There are total 40 questions in the exercise of this chapter. We have written the solution of all the questions in very simple language with proper explanation. This solution notes will help very much to increase the comprehensive knowledge in organic chemistry. Hence, you must read till end.
In Ncert Solution of Organic Chemistry, we have to read about the basic concepts of organic chemistry. This chapter explains in details about the basic terms, types of organic reactions, mechanism of reactions applied in organic chemistry, structure and hybridisation of organic compounds. We also learn about the basic principles and techniques of isolation of elements in organic compounds. This chapter enhance the knowledge of students to a great extent so much that students feel easy and interesting in study the whole syllabus of organic chemistry. Hence, we must read this chapter over and again.
Ncert Solution of Organic Chemistry Class 11: Overview
Some Basic Principles and Techniques of organic chemistry gives us a learning attitude and activities about chemical equations in organic chemistry. We have a lot of things to learn in this chapter. Let us know about them:
- To understand reasons for tetravalency of carbon and shapes of organic molecules.
- To write structures of organic molecules in various ways.
- To classify the compounds.
- To name the compounds according to IUPAC system and also derive their structure from the given names.
- To understand the concept of organic reaction mechanism.
- To understand the influence of electronic displacements on structure and reactivity of organic compounds.
- To recognize the types of organic reactions.
- To learn the techniques of purification of organic compounds.
- To write the chemical reactions involved in the qualitative analysis of organic compounds.
- To understand the principles involved in quantitative analysis of organic compounds.
Questions and answers of Ncert Solutions of Organic Chemistry:
Question 1. What are hybridisation states of each carbon atom in the following compounds? CH2=C=O, CH3CH=CH2, (CH3)2CO, CH2=CHCN, C6H6. (Ncert Solution of Organic Chemistry)
Answer: 
Question 2. Indicate the σ- and π-bonds in the following molecules:
C6H6 , C6H12, CH2Cl2, CH=C=CH2, CH3NO2, HCONHCH3
Answer: 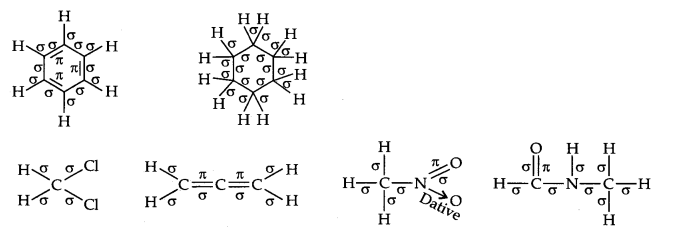
Question 3. Write bond-line formulas for: Isopropyl alcohol, 2,3-Dimethylbutanal, Heptan-4-one. (Ncert Solution of Organic Chemistry)
Answer: 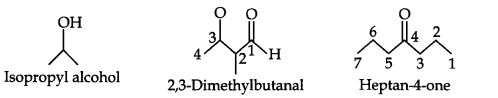
Question 4. Give the TUPAC names of the following compounds: 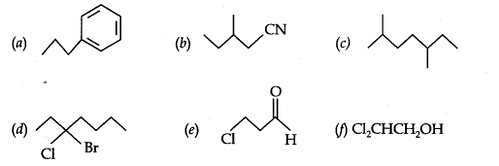
Answer: (a) propylbenzene (b) 3- Methyl pentanitrile (c) 2,5 – Dimethylheptane (d) 3 – Bromo – 3 – chloroheptane (e) 3- chloro propanal (f) 2,2- Dichloroethanol.
Question 5. Which of the following represents the correct IUPAC name for the compounds concerned? (Ncert Solution of Organic Chemistry)
(a) 2, 2-Dimethylpentane or 2-Dimethylpentane (b) 2, 4, 7-Trimethyloctane or 2, 5, 7- Trimethyloctane (c) 2-Chloro-4-methylpentane or 4-Chloro-2-methylpentane (d) But-3-yn- l-ol or But-4-ol-yne.
Answer: (a) 2, 2-Dimethylpentane (b)2, 4, 7-Trimethyloctane. For two alkyl groups on the same carbon its locant is repeated twice, 2, 4, 7-locant set is lower than 2, 5, 7. (c) 2- Chloro-4-methylpentane. Alphabetical order of substituents, (d) But-3-yn-l-ol. Lower locant for the principal functional group, i.e., alcohol.
Question 6. Draw formulas for the first five members of each homologous series beginning with the following compounds, (Ncert Solution of Organic Chemistry)
(a) H—COOH (b) CH3COCH3 (c) H—CH=CH2
Answer: (a) CH3—COOH
CH3CH2—COOH, CH3CH2CH2—COOH
CH3CH2CH2CH2—COOH, CH3CH2CH2CH2CH2—COOH
(b) CH3COCH2CH3
CH3CH2COCH2CH3, CH3COCH2CH2CH3
CH3COCH2CH2CH2CH3
CH3CO(CH3)4CH3
(c) H—CH=CH2
CH3CH=CH2
CH3CH2CH=CH2
CH3CH2CH2CH=CH2
CH3CH2CH2CH2CH=CH2
Question 7. Give condensed and bond line structural formulas and identify the functional group(s) present, if any, for: (a) 2, 2, 4-Trimethylpentane (b) 2-Hydroxy-l, 2, 3-propanetricarboxylic acid (c) Hexanedial.
Answer: 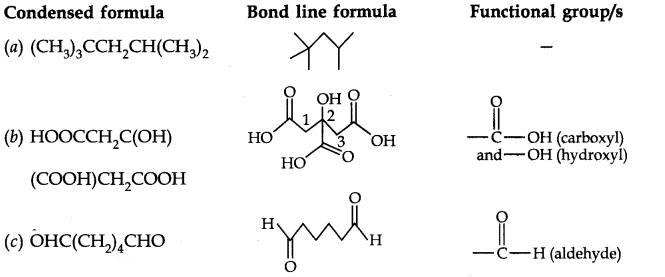
Question 8. Identify the functional groups in the following compounds: 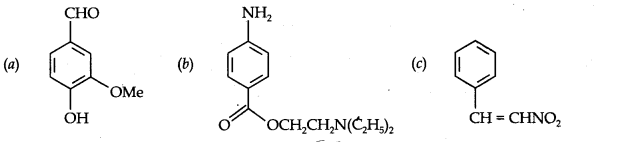
Answer: 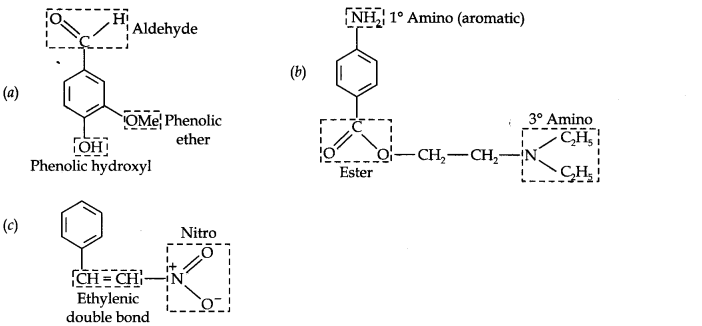
Question 9. Which of the two: O2NCH2CH2O– or CH3CH2O– is expected to be more stable and why? (Ncert Solution of Organic Chemistry)
Answer: O2N——<——CH2——<——CH2 —<—— O– is more stable than CH3——<——CH2——<——O- because NO2 group has -I-effect and hence it tends to disperse the -ve charge on the O-atom. In contrast, CH3CH2 has +I-effect. It, therefore, tends to intensify the -ve charge and hence destabilizes it.
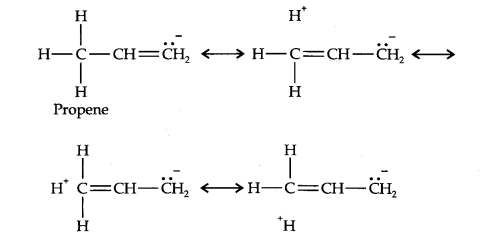
Question 10. Explain why alkyl groups act as electron donors when attached to a π-system.
Answer: Due to hyperconjugation, alkyl groups act as electron donors when attached to a π- system as shown below:
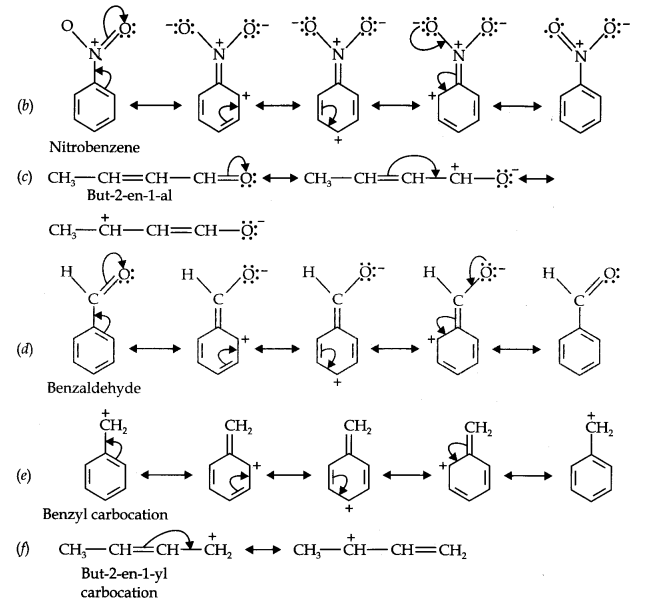
Question 11. Draw the resonance structures for the following compounds. Show the electron shift using curved-arrow notation. (a) C6H5OH (b) C6H5N02 (c) CH3CH=CHCHO (d) C6H5—CHO (e) C6H5—CH2 (f) CH3CH=CHCH2
Answer: 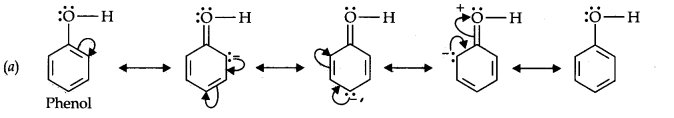
Question 12. What are electrophiles and nucleophiles? Explain with examples: (Ncert Solution of Organic Chemistry)
Answer: Electrophiles: Electrons loving species are called electrophiles. They are electron deficient neutral molecules or positive charge carrying ions. Examples are H+, Cl+, Br+, NO2+, R3C+, RN2+, AlCl3, BF3 etc.
Nucleophiles: Nucleus loving species are called nucleophiles. They are electron rich neutral molecules or negative charge carrying ions. For example Cl– Br–, CN–, OH–, RCR2–, NH3, RNH2, H2O, ROH etc.
Question 13. Identify the reagents shown in bold in the following equations as nucleophiles or electrophiles (Ncert Solution of Organic Chemistry)
(a) CH3COOH + HO– → CH3COO– + H2O
(b) CH3COCH3 + CN– → (CH3)2 C(CN)(OH)
(c) C6H5 + CH3CO+ → C6H5COCH3
Answer: Reagents of (a) and (b) are nucleophiles and of (c) is electrophile.
Question 14. Classify the following reactions in one of the reaction type studied in this unit.
(a) CH3CH2Br + HS– → CH3CH2SH + Br–
(b) (CH3)2C=CH2 + HCl → (CH3)2CCl—CH3
(c) CH3CH2Br + HO– → CH2=CH2 + H2O + Br–
(d) (CH3)3C—CH2OH + HBr → (CH3)2 C Br CH2CH2CH3 + H2O
Answer: (a) Nucleophilic substitution reaction (b) Electrophilic addition reaction
(c)Bimolecular elimination reaction (d) Nucleophilic substitution with rearrangement reaction.
Question 15. What is the relationship between the members of following pairs of structures? Are they structural or geometrical isomers or resonance contributors? 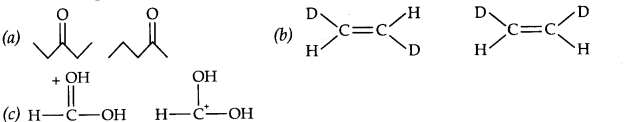
Answer: (a) These pair reflect position isomerism as well as metamerism. (b) These pair show geometrical isomerism (c) These pair show resonance.
Question 16. For the following bond cleavages, use curved-arrows to show the electron flow and classify each as homolysis or heterolysis. Identify reactive intermediate produced as free radical, carbocation and carbanion. 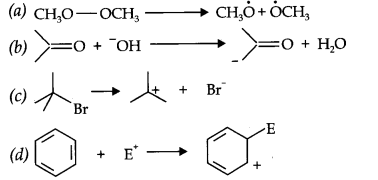
Answer: 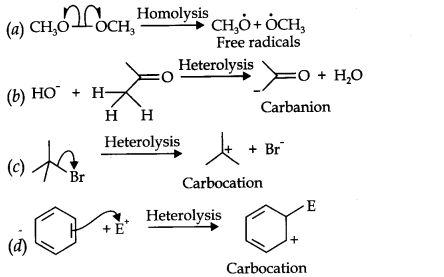
Question 17. Explain the terms inductive and electromeric effects. Which electron displacement effect explain the following correct orders of acidity of the carboxylic acids?
(a) Cl3CCOOH > Cl2CHCOOH > ClCH2 COOH
(b) CH3CH2COOH > (CH3)2 CHCOOH > (CH3)3CCOOH
Answer: Inductive effect: The permanent displacement of σ- electrons along a carbon chain whenever an electron withdrawing or donating group is present at the end of the chain is called Inductive effect. There are two types of Inductive effects:
- + I – effect: If the substituent attached to the end of the carbon chain is electron donating, the effect is called +I- effect.
- –I- effect: If the substituent linked at the end of the carbon chain is electron withdrawing, the effect is called –I- effect.
- Examples of -I effect are: –NO2, –F, –Cl, –Br, –I, –OH etc.
- Examples of +I effect are (Electron releasing)
(CH3)3C— , (CH3)2CH—, CH3CH2— CH3— etc.
Electromeric effect: In this defect, there is complete transfer of π- electrons of a multiple bond to one of the bonded usually more electronegative atom in presence of an attacking reagent. It is also called E- effect. There are two types of +E effect: (Ncert Solution of Organic Chemistry)
- If the electrons of the π- bond are transferred to that atom of the double bond to which the reagents finally attached, the effect is called + E- effect.
- If the electrons of the double bond are transferred to an atom of the double bond other than the one to which the reagents gets finally attached, the effect is called –E- effect.
(a) As the number of halogen atoms decreases, the overall –I- effect decreases and hence the acidic strength decreases. 
(b) As the number of alkyl group increases, the + I- effect increases and hence the acidic strength decreases. 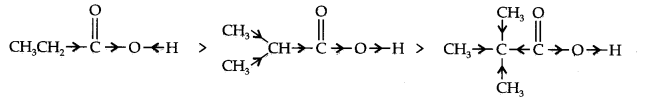
Question 18. Give a brief description of the principles of the following techniques taking an example in each case: (a) Crystallisation (b) Distillation (c) Chromatography
Answer: (a) Crystallisation: The process by which an impure compound is converted into its crystals is called crystallisation. This method is used for purification of solid organic compounds. It is based on the difference in the solubilities of the compound and the impurities in a suitable solvent. For example: separation of benzoic acid from naphthalene. (Ncert Solution of Organic Chemistry)
(b) Distillation: This method involves the conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling. This method is used for those liquids which are sufficiently stable at their boiling points and which contain non- volatile impurities. For example, benzene, ethanol, acetone can be purified by this method.
(c) Chromatography: The technique of separating the components of a mixture in which separation is done by the differential movement of individual components through a stationary phase under the influence of a mobile phase. The stationary phase can be either a solid or tightly bound liquid on a solid support while the mobile phase can be either a liquid or a gas. This method is widely used for separation, purification and identification of the components of a mixture, weather coloured or colourless. (Ncert Solution of Organic Chemistry)
Question 19. Describe the method, which can be used to separate two compounds with different solubilities in a solvent S.
Answer: Two compounds with different solubilities in a solvent S can be separated by fractional crystallisation. When a hot saturated solution of these two compounds is allowed to cool, the less soluble compound crystallises out first while the more soluble remains in the solution. The crystals were separated from the mother liquor and mother liquor is again concentrated and the hot solution again allowed to cool when the crystals of the second compound are obtained. These are again filtered and dried.
Question 20. What is the difference between distillation, distillation under reduced pressure and steam distillation? (Ncert Solution of Organic Chemistry)
Answer: Distillation: It involves conversion of a liquid into vapours followed by condensation of the vapours thus produced by cooling to get the pure liquid while the non- volatile impurities remain in the flask. This method is commonly used for those liquids which are sufficiently stable at their boiling points and contain non- volatile impurities.
Distillation under reduced pressure: It involves conversion of a liquid into vapours by heating followed by condensation of the vapours thus produced by cooling but the pressure acting on the system is not atmospheric but is reduced by using a vacuum pump. Since the boiling point of a liquid decreases as the pressure acting on it is reduced, therefore, this method is used to purify such liquids have high boiling liquids or liquid which decompose at or below their boiling points. (Ncert Solution of Organic Chemistry)
Steam distillation: There is no reduction in the total pressure acting on the solution. Here, the mixture of organic liquid and water boils at a temperature when the sum of the vapour pressure of the organic liquid (p1) and that of water (p2) becomes equal to the atmospheric pressure (p) = p1 + p2
Since the vapour pressure of water around its boiling point is quite high and that of the liquid is quite low, therefore, the organic liquid will boil at a temperature much lower than its normal boiling point and hence its decomposition is avoided. Steam distillation is used to purify such liquids which are volatile in steam, insoluble in water, possesses a vapour pressure of about 10.5 mm of Hg and contain non- volatile impurities. (Ncert Solution of Organic Chemistry)
Questions 21. Discuss the Chemistry of Lassaigne’s test.
Answer: This is the most reliable test for detecting nitrogen, sulphur and halogens in an organic compound. In this test, the elements present in the organic compound are converted from covalent form into Ionic form by fusing the compound with sodium metal.
In this test, A small piece of freshly pea size cut of sodium is heated gently in a fusion tube till it forms a shining globule. The tube is removed from the flame and a small amount of the organic compound is added and heated strongly till it becomes red hot. Now the hot tube is plunged into a China dish containing 10 – 15 ml of distilled water. The contents of China dish are boiled for a few minutes, cooled and then filtered. This filtrate is called Lassaigne’s extract or sodium fusion extract. (Ncert Solution of Organic Chemistry)
Question 22. Differentiate between the principle of estimation of nitrogen in an organic compound by (1) Dumas method (ll) Kjeldahi’s method.
Answer: (1) Dumas method: In this method, a known mass of the organic compound is heated with excess of CuO in an atmosphere of CO2, when nitrogen of the organic compound is converted into N2 gas. The volume of N2 thus obtained is converted into NTP and the percentage of nitrogen is determined by applying the formula.
% of N = (28/22400) × (vol. of N2 at NTP / mass of the substance taken) × 100.
(ll) Kjeldahi’s method : In this method, the known mass of the organic compound is heated with conc. H2SO4 in presence of K2SO4 and little CuSO4 in a long necked flask called Kjeldahi’s flask. When nitrogen present in the organic compound is quantitatively converted into (NH4)2SO4. Now this sulphate is boiled with excess of NaOH solution to liberate NH3 gas which is absorbed in a known excess of a standard acid as H2SO4 or HCl. (Ncert Solution of Organic Chemistry)
The volume of acid is found by titration against a standard alkali solution. From the volume of acid, the percentage of nitrogen is determined by applying the equation: % N = 1.4 × Molarity of the acid × Basicity of the acid × volume of acid used/Mass of the substance taken.
Question 23. Discuss the principle of estimation of halogens, sulphur and phosphorus present in an organic compound.
Answer: Estimation of halogens: A known mass of the organic substance is heated with fuming nitric acid and a few crystals of silver nitrate in a sealed tube called carius tube in a furnace. Under the conditions, carbon and hydrogen are oxidised to carbon dioxide and water while halogen is converted into silver halide. The precipitate of AgNO3 is filtered, dried and weighed. With the help of the mass of AgNO3, the percentage of halogen is calculated as:
% of halogen = Atomic mass of X / (108 + Atomic mass of X) × mass of AgX × (100/mass of the substance taken).
Estimation of sulphur: A known mass of the substance is heated with sodium peroxide or fuming Nitric acid in a sealed tube (carius tube). Carbon and hydrogen are oxidised to CO2 and H2O while sulphur is oxidised to Sulphuric acid which is then precipitated as barium sulphate by adding excess of barium chloride solution. The ppt of BaSO4 is filtered, dried and weighed. Knowing the mass of BaSO4, the percentage of sulphur is estimated with the help of the following equation.
% of S = (32/233) × (mass of BaSO4 formed/ mass of the substance taken) ×100.
Estimation of Phosphorous: A known mass of the substance is heated with fuming Nitric acid in a sealed tube (carius tube). Carbon and hydrogen are oxidised to CO2 and H2O while Phosphorous is oxidised to phosphoric acid which is then precipitated as ammonium phosphomolybdate by heating it with conc HNO3 and then adding ammonium molybdate. The ppt of ammonium phosphomolybdate is filtered, washed, dried and weighed. Knowing the mass of ammonium phosphomolybdate, the percentage of Phosphorous is estimated with the help of the following equation.
% of P = (31/1877) × (Mass of amm. phosphomolybdate/Mass of the substance taken) × 100.
Question 24: Explain the principle of paper chromatography. (Ncert Solution of Organic Chemistry)
Answer: paper chromatography is a type of partition chromatography. In paper chromatography, a special type of paper is used which consists of cellulose. The water absorbed on this paper behave as stationary phase. The mobile phase is another liquid which is usually a mixture of two or more solvents with water as one of the components. It is based upon continuous differential partitioning of the various components of the mixture between the stationary phase and the mobile phase.
Question 25. Why is Nitric acid added to sodium extract before adding Silver nitrate for testing halogens?
Answer: Sodium extract is boiled with nitric acid to decompose NaCN and Na2S NaCN + HNO3 → NaNO3 + HCN ↑ , Na2S + 2HNO3 → 2NaNO3 + H2S ↑
,if present, otherwise these will react with AgNO3 and hence will interfere with the test as shown below: NaCN + AgNO3 → AgCN(white ppt) + NaNO3, Na2S + 2AgNO3 → Ag2S (black ppt) + 2 NaNO3.
Question 26. Explain the reason for the fusion of an organic compound with metallic sodium for testing nitrogen, sulphur and halogens. (Ncert Solution of Organic Chemistry)
Answer: The organic compound is fused with sodium metal to convert these elements which are present in the covalent form to ionic form.
Question 27. Name a suitable technique of separation of the components from a mixture of calcium sulphate and camphor.
Answer: A mixture of CaSO4 and camphor can be separated by the following two methods:
(1) By sublimation because camphor is volatile but CaSO4 is not, therefore sublimation of the mixture gives camphor on the sides of funnel while CaSO4 is left in the china dish.
(ll) Camphor is soluble in organic solvents like CHCl3, CCl4 etc. While CaSO4 is not. Therefore, when the mixture is shaken with the solvent, camphor goes into solution while CaSO4 remains as residue. It is filtered and evaporation gives camphor.(Ncert Solution of Organic Chemistry)
Question 28. Explain why an organic liquid vaporise at a temperature below its boiling point in its steam distillation?
Answer: In steam distillation, the mixture consisting of the organic liquid and water boils at a temperature when the sum of the vapour pressure of the liquid (p1) and that of water (p2) becomes equal to the atmospheric pressure, i.e., p = p1 + p2.
Since, the vapour pressure of water around the boiling point of the mixture is quite high and that of liquid is quite low (10 – 15 mm), therefore the organic liquid distills at a pressure much lower than the atmospheric pressure. (Ncert Solution of Organic Chemistry)
Question 29. Will CCl4 give white ppt of AgCl on heating with silver nitrate? Give reason for your answer. (Ncert Solution of Organic Chemistry)
Answer: When CCl4 is heated with AgNO3 solution, white ppt of AgCl will not be formed. The reason being that CCl4 is a covalent compound, therefore, it does not ionize to give Cl– ions needed for the formation of the ppt of AgCl.
Question 30. Why is a solution of Potassium hydroxide used to absorb carbon dioxide evolved during the estimation of carbon present in an organic compound?
Answer: CO2 is acidic in nature, therefore, it reacts with the strong base KOH to form K2CO3. The increase in the mass of U- tube containing KOH then gives the mass of CO2 produced and from its mass the percentage of carbon in the organic compound is estimated.
Question 31. Why is it necessary to use acetic acid and not Sulphuric acid for acidification of sodium extract for testing sulphur by lead acetate test? (Ncert Solution of Organic Chemistry)
Answer: For testing sulphur, the sodium extract is acidified with acetic acid because lead acetate is soluble and does not interfere with the test. If H2SO4 were used, lead acetate itself will react with H2SO4 to form white ppt of lead sulphate which will interfere with the test. Pb(OCOCH3)2 + H2SO4 → PbSO4 ↓ + 2CH3COOH.
Question 32. An organic compound contains 69% carbon and 4.8% hydrogen, the remainder being oxygen. Calculate the mass of carbon dioxide and water produced when 0.20 g of this substance is subjected to complete combustion.
Answer. We know that, % of C = (12/44) × (Mass of CO2 formed / Mass of the substance taken) × 100, Now, substituting the % of C and the mass of the substance taken, we have 69 = (12/44) × (mass of CO2 formed/ 0.20) × 100 ∴ mass of CO2 formed = (69 × 44 × 0.20)/(12 × 100) = 0.506 g.
Similarly, % of H = (2/18) × (mass of H2O formed /mass of substance taken) × 100. Substituting the % of H and mass of the substance taken, we have, 4.8 = (2/18) × (mass of H2O formed/0.20) × 100 or Mass of H2O formed = (4.8 × 18 × 0.20)/ 2 × 100 = 0.0864 g.
Question 33. A sample of 0.50 g of an organic compound was treated according to Kjeldahi’s method. The ammonia evolved was absorbed in 50 mL of 0.5 M H2SO4. The residual acid required 60 mL of 0.5 M solution of NaOH for neutralization find the percentage composition of nitrogen in the compound.
Answer: Step 1. To determine the volume of H2SO4 used. Volume of acid taken = 50 ml of 0.5 M H2SO4 = 25 ml of 1 M H2SO4. Volume of alkali used for neutralization of excess acid = 60 ml of 0.5 NaOH = 30 ml of 1 M NaOH. Now 1 mole of H2SO4 neutralizes 2 moles of NaOH (i.e., H2SO4 + 2NaOH → Na2SO4 + 2H2O). ∴ 30 ml of 1 M NaOH = 15 ml of H2SO4 ∴ Volume of acid acid used by ammonia = 25 – 15 = 10 ml.
Step 2. To determine the percentage of nitrogen. % of N = (1.4 × Molarity of the acid × Basicity of the acid × volume of acid used )/Mass of the substance taken. Substituting the value of all the terms, we have, % of N = (1.4 × 1 × 2 × 10)/0.5 = 56.0 %.
Question 34. 0.3780 g of an organic chloro compound gave 0.5740 g of Silver chloride in carius estimation. Calculate the percentage of chlorine present in the compound.
Answer: Here, the mass of the substance taken = 0.3780 g, Mass of AgCl formed = 0.05740 g. Now 1 mole of AgCl = 1 g atom of Cl or 143.5 g of AgCl = 35.5 g of Cl ∴ applying the relation, % of Cl = (35.5/143.5) × (Mass of AgCl formed / Mass of the substance taken) × 100 = (35.5 /143.5) × (0.5740/0.3780) × 100 = 37.566%
Question 35. In the estimation of sulphur by carius method, 0.468 g of an organic sulphur compound afforded 0.668 g of barium sulphate. Find the percentage of sulphur in the given compound. (Ncert Solution of Organic Chemistry)
Answer: Here, the mass of the substance taken = 0.468 g, Mass of BaSO4 formed = 0.668 g. Now 1 mole of BaSO4 = 1 g atom of S or 233 g of BaSO4 = 32 g of S. Applying the relation of estimation for S, % of S = (32/233) × (Mass of BaSO4 formed/ Mass of the substance taken) × 100 = (32/233) × (0.668/0.468) × 100 = 19.6%.
Question 36. In the organic compound CH2 = CH – CH2 – CH2 – C ≡ CH, the pair of hybridised orbitals involved in the formation of C2 – C3 bond is:
(a) sp – sp2 (b) sp – sp3 (c) sp2 – sp3 (d) sp3 – sp3
Answer: When both double and triple bonds are present in an organic compound, double bond is given preference while numbering the carbon chain. Hence, C2 – C3 bond is formed by overlap of sp2 – sp3 orbitals. Thus, option (c) is correct.
Question 37. In the Lassaigne’s test for nitrogen in an organic compound, the prussian blue colour is obtained due to the formation of: (a) Na4[Fe(CN)6] (b) Fe4[Fe(CN)6]3 (c) Fe2[Fe(CN)6] (d) Fe3[Fe(CN)6]4
Answer: The prussian blue colour is due to the formation of Fe4[Fe(CN)6]3. Thus, option (b) is correct.
Question 38. Which of the carbocation is most stable? (Ncert Solution of Organic Chemistry)
(a) (CH3)3CCH2+ (b) (CH3)3C+ (c) CH3CH2CH2+ (d) CH3CH+CH2CH3
Answer: The order of stability of carbocation is: 3o > 2o > 1o. Thus, option (b) is correct.
Question 39. The best and latest technique for isolation, purification and separation of organic compounds is: (a) crystallisation (b) distillation (c) sublimation (d) chromatography
Answer: chromatography. Thus, option (d) is correct (Ncert Solution of Organic Chemistry)
Question 40. The reaction: CH3CH2I + KOH (aq) → CH3CH2OH + KI is classified as: (a) electrophilic substitution (b) nucleophilic substitution (c) elimination (d) addition.
Answer: This is an example of nucleophilic substitution reaction since the nucleophile I– is replaced by the nucleophile OH– ion. Thus, option (b) is correct.
Conclusion of Ncert Solution of Organic Chemistry
Ncert Solution of Organic Chemistry provides the solution of all the questions illustrated in the exercise of Ncert book of chemistry class 11th. We hope that you all have been satisfied and learnt the answer of the given questions. If you have any doubts or suggestions, please comment me at the comment section.
You all are further requested to share this solution notes among your friends and relatives. We shall be very grateful to you for this kind attention.

