Ncert solutions of d and f block pdf: Here we shall learn to solve all the questions asked in intext and exercise of this chapter. The elements of this block are also known as transition elements and inner transition elements respectively. All the elements of this group show variable oxidation state and properties. The elements of these blocks play very important roles in our life. You all should have a good knowledge of this chapter.
The d-block elements are those whose last electrons enters the d- orbitals of valence shell. For example Sc, Ti, V, Cr etc. The f- block elements are those whose last electron enters the f-orbtals of their valence shell. For example Ce, Pr, Mo, Sm, Th, Pd etc. If you want know in detail about this chapter. You can visit on the post “Notes of d and f block elements “.
Here we have discussed the Ncert solutions of d and f block elements. After reading out this solution you can have good command all over this chapter. This is very important for the entrance exams and also for other competitive exams. Let us go through the Learning Objectives of this chapter.
Learning Objectives of Ncert solutions of d and f block elements:
- To learn the positions of the d and f block elements in the periodic table.
- To know about the electronic configuration of the transition and inner transition elements.
- To explain the relative stability of various oxidation states in terms of electrode potential values.
- To describe the preparation, properties, structures and Uses of important compounds such as K2Cr2O7 and KMnO4.
- To understand the general characteristics of the d and f block elements and their general trends in groups and periods.
- To describe the properties of the f block elements and give a comparative account of the Lanthanoids and actinoids with respect to their electronic configuration, oxidation states and chemical behaviour.
Ncert solutions of d and f block: Intext questions
Question 1. Silver atoms has completely filled d-orbitals (4d10) in its ground state. How can you say that it is a transition element?
Answer: The outer electronic configuration of Ag (Z= 47) is 4d10 5s1. In addition to +1, it shows an oxidation state of +2. In +2 oxidation state, the configuration is d9 that means it has incompletely filled d- subshell. Hence, it is a transition element.
Question 2. In the series Sc (Z = 21) to Zn (Z = 30), the enthalpy of atomisation of zinc is the lowest, i.e., 126 KJmol-1. Why?
Answer: In the series of Sc to Zn, all elements have one or more unpaired electrons except zinc which has no unpaired electrons in its outer electronic configuration as 3d10 4s2. Hence, atomic intermetallic bonding is weakest in zinc. Therefore, enthalpy of atomisation is lowest.
Question 3. Which of the 3d series of the transition metals exhibits the largest number of oxidation states and why?
Answer: Manganese (Z = 25) shows maximum number of oxidation states. This is because its electronic configuration is 3d5 4s2. As 3d and 4s are close in energy. It has maximum number of electrons to lose or share as all the 3d electrons are unpaired. Hence, it shows maximum number of oxidation states from +2 to +7.
Question 4. Eo (M 2+ /M) for copper is positive (+ 0-34 V). What is possibly the reason for this?
Answer: Eo( M2+/M) for any metal is related to the sum of the enthalpy changes taking place in the following steps:
M(s) + ΔaH → M(g), (ΔaH = enthalpy of atomisation) M(g) + ΔiH → M 2+(g ), ( ΔiH = ionization enthalpy) M2+ (g) + aq → M 2+ (aq) + ΔhydH ( ΔhydH = hydration enthalpy). Copper has high enthalpy of atomisation (i.e., energy absorbed) and low enthalpy of hydration, energy (i.e. released). Hence, Eo(Cu2+/Cu) is positive.
The high energy required to transform Cu (s) to Cu2+ (aq) is not balanced by its hydration enthalpy.
Question 5. How would you account for the irregular variation of ionization enthalpies (first and second) in series of the transition elements?
Ans. Irregular variation of first ionization enthalpy. As we move from left to right along the first transition series, as effective nuclear charge increases, it is expected in general that the first ionization enthalpy should show an increasing trend. However, the trend is irregular because removal of the electron alters the relative energies of 4 s and 3 d orbitals. Thus, there is a reorganisation energy accompanying ionization.
This results into the release of exchange energy which increases as the number of electrons increases in the d” configuration and also from the transference of s- electrons into d-orbitals. Cr has low first ionization energy because loss of one electron gives stable electronic configuration (3d5). Zn has very high ionization energy because electron has to be removed from 4 s orbital of stable configuration (3d10 4s2).
Irregularities of second ionization enthalpy. After the loss of one electron, the removal of second electron becomes difficult. Hence, second ionization enthalpies are much higher and in general increase from left to right. However, Cr and Cu show much higher values because the second electron has to be removed from the stable configurations of Cr+ (3d5) and Cu+ (3d10).
Question 6. Why is the highest oxidation state of a metal exhibited in its oxide or fluoride only?
Answer: Oxygen and Fluorine have small size and high electronegativity. Hence, they can oxidise the metal to the highest oxidation state.
Question 7. Which is a stronger reducing agent Cr2+ or Fe2+ and why?
Answer: Cr2+ is a stronger reducing agent than Fe2+ because EoCr3+/Cr2+ is – ve (– 0.41 V) whereas EoFe3+/Fe2+ is + ve (+0.77 V). Thus Cr2+ is easily oxidised to Cr3+ but Fe2+ does not do so. Hence, Cr2+ is stronger reducing agent than Fe3+.
Question 8. Calculate the ‘spin only’ magnetic moment of M2+(aq) ion (Z= 27)
Answer: 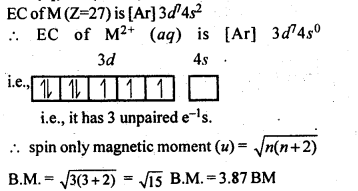
Question 9. .Explain why Cu+ ion is not stable in aqueous solutions?
Answer: Cu2+(aq) is much more stable than Cu+(aq). This is because although second ionisation enthalpy of copper is large but ΔhydH for Cu2+(aq) is much more negative than that for Cu+(aq) and hence it is more than compensate for the second ionization enthalpy of copper. Therefore, copper (I) compounds are unstable in aqueous solution and undergo disproportionation as 2Cu+ → Cu2+ + Cu.
Question 10. Actinoid contraction is greater from element to element than lanthanoid contraction. Why?
Answer: This is due to poor shielding by 5 f electrons in the actinoids than that by 4 f electrons in the lanthanoids.
Answers of Exercise questions in Ncert solutions of d and f block
Question 1: Write down the electronic configuration of (i) Cr3+ (ii) Pm3+ (iii) Cu+ (iv) Ce4+(v) Co2+ (vi) Lu2+(vii) Mn2+ (viii) Th4+.
Answer:(i) Cr3+ = [Ar]183d3
(ii)Pm3+ = [Xe]54 4f4
(iii)Cu+ = [Ar]18 3d10
(iv)Ce4+ = [Xe]54
(v)Co2+ = [Ar]18 3d7
(vi)Lu2+ = [Xe]54 4f14 5d1
(vii) Mn2+ = [Ar]18 3d5 (viii)Th4+= [Rn]86
Question 2. Why are Mn2+ compounds more stable than Fe2+ towards oxidation to their+3 state?
Answer: Electronic configuration of Mn2+ is 3d5 which is half-filled and hence stable. Therefore, 3rd oxidation enthalpy is very high that means 3rd electron can not be lost easily. In case of Fe2+, electronic configuration is 3d6. Hence, it can lose one electron easily to give the stable configuration 3d5.
Question 3. Explain briefly how+2 state becomes more and more stable in the first half of the first row transition elements with increasing atomic number?
Answer: Except scandium (which shows oxidation state +3), all other first rows transition elements show an oxidation state of +2. This is due to loss of two 4s electrons. In the first half, as we move from Ti2+ to Mn2+, the electronic configuration changes from 3d2 to 3d5 that means more and more of d-orbitals are half-filled imparting greater stability to +2 state.
In the second half that means Fe2+ to Zn2+, the electronic configuration changes from 3d6 to 3d10 which implies that 3d orbitals pair up and the number of half-filled orbitals decreases. Hence, the stability of +2 state decreases.
Question 4. To what extent do the electronic configurations decide the stability of oxidation states in the first series of the transition elements? Illustrate your answer with examples.
Answer: In a transition series, the oxidation states which lead to noble gas or exactly half filled or completely filled d-orbitals are more stable. For example, in the first, electronic configuration of Mn (Z = 25) is [Ar] 3d5 4s2. It shows oxidation states +2 to +7 but Mn(II) is most stable because it has the half-filled configuration [Ar]3d5. Similarly Sc3+ and Zn2+ are more stable.
Question 5. What may be the stable oxidation state of the transition element with the following d electron configurations in the ground state of their atoms: 3d3,3d5, 3d8 and 3d4?
Answer: 3d3 4s1 = + 5.
3d5 4s2 = + 2, + 7, 3d5 4s1 =+6.
3d84s2 = + 2.
3d44s2 = 3d5 4s1 = + 6(and + 3). Note. The maximum oxidation states of reasonable stability correspond to the sum of s and d electrons upto Mn. After that, there is abrupt decrease in the stability of higher oxidation states.
Question 6. Name the oxometal anions of the first transition series of the transition metals in which the metal exhibits the oxidation state equal to its group number.
Answer: Cr2O72– and CrO42– (Group No = 6 and oxidation state of Cr = 6). MnO42– (Group number = 7 and oxidation state = 7).
Question 7. What is lanthanoid contraction? What are the consequences of lanthanoid contraction?
Answer:
Lanthanoid Contraction refers to the gradual decrease in the size of atoms (or ions) of the lanthanoid elements as you move from lanthanum (La) to lutetium (Lu) in the periodic table. These elements are found in the f-block, from atomic number 57 to 71.
This size decrease happens because, as we go across the lanthanoid series, more protons are added to the nucleus. The increased positive charge pulls the electrons closer to the nucleus, but the added inner f-electrons don’t shield the outer electrons effectively from this pull. As a result, the size of the atoms keeps shrinking.
Consequences of Lanthanoid Contraction:
1. Similar sizes of post-lanthanide elements: Elements like zirconium (Zr) and hafnium (Hf), or niobium (Nb) and tantalum (Ta), which are in the d-block, have very similar atomic and ionic sizes because of the lanthanoid contraction. This makes their chemical properties quite similar.
2. Higher density and hardness: As the size of the atoms decreases, the elements become denser and harder.
3. Chemical properties: It influences the chemical reactivity and coordination numbers of the lanthanoids and affects the properties of elements beyond the lanthanoids in the periodic table.
Question 8. What are the characteristics of the transition elements and why are they called transition elements? Which of the d block elements may not be regarded as the transition elements?
Answer: Characteristics of Transition Elements:
1. Variable oxidation states: Transition elements can show different oxidation states.
2. Formation of colored compounds: Many compounds of transition elements are colored.
3. Formation of complexes: They easily form coordination compounds or complexes.
4. Magnetic properties: Some of them are magnetic due to unpaired electrons.
5. Good conductors: They are good conductors of heat and electricity.
6. Catalytic activity: Many transition elements act as catalysts in chemical reactions.
Why are they called Transition Elements?
They are called transition elements because they form a “transition” between the main group elements of the periodic table. Their properties are intermediate between the s-block and p-block elements.
d-block Elements Not Regarded as Transition Elements:
Zinc (Zn), Cadmium (Cd), and Mercury (Hg) are not usually regarded as transition elements because they have a completely filled d-orbital in their most common oxidation state (Zn²⁺, Cd²⁺, Hg²⁺).
Question 9. In what way is the electronic configuration of transition elements different from that of the non- transition elements?
Answer: Transition elements contain incompletely filled d-subshell and have electronic configuration (n – 1)d1–10 ns0–2. Whereas non- transition elements have no d-subshell or their d-subshell is completely filled and have ns1–2 or ns2 np1–6 in their outer most shell.
Question 10. What are different oxidation states exhibited by Lanthanoids?
Answer: The most common oxidation states of lanthanoids is +3. However, some lanthanoids also show an oxidation state of +2 and +4. For example, Eu shows an oxidation state of +2 and Ce shows an oxidation state of +4.
Question 11. Explain giving reasons:
(i)Transition metals and many of their compounds show paramagnetic behaviour.
(ii)The enthalpies of atomisation of the transition metals are high.
(iii)The transition metals generally form coloured compounds.
(iv)Transition metals and their many compounds act as good catalyst
Answer: (i) Paramagnetic behaviour: Transition metals and many of their compounds are paramagnetic because they have unpaired electrons in their d-orbitals. Unpaired electrons create a magnetic field, making them attracted to external magnetic fields.
(ii) High enthalpies of atomisation: The enthalpies of atomisation of transition metals are high because the metallic bonds between the atoms are very strong. This strength comes from the involvement of both s and d electrons in bonding, requiring more energy to break these bonds.
(iii) Coloured compounds: Transition metals form coloured compounds because their d-electrons can absorb certain wavelengths of light and jump between different energy levels. The light that is not absorbed is reflected, giving the compound its color.
(iv) Catalytic behaviour: Transition metals and their compounds act as good catalysts because they can easily lend and accept electrons due to their variable oxidation states. This ability allows them to facilitate reactions without being consumed.
Question 12. What are interstitial compounds? Why are such compounds well known for transition metals?
Answer: Those compounds which are formed when small atoms like hydrogen, carbon, or nitrogen fit into the spaces (interstices) between the metal atoms in a crystal lattice are called Interstitial compounds. For example TiC,TiH2, Mn4N.
Transition metals are well known for forming interstitial compounds because they have large atomic sizes and gaps in their crystal structure, allowing these small atoms to occupy the spaces without significantly disturbing the metal lattice. These compounds often enhance the hardness and strength of the metals without changing their chemical properties much.
Questions 13. How is the variability in oxidation states of transition metals different from that of the non-transition metals? Illustrate with examples.
Answer: The variability in oxidation states of transition metals is greater than that of non-transition metals. This happens because transition metals have both s and d-electrons available for bonding, allowing them to show multiple oxidation states. In contrast, non-transition metals usually only use their s and p-electrons, so they have fewer oxidation states.
For Examples: Transition metals like Iron (Fe) can show oxidation states of +2 (Fe²⁺) and +3 (Fe³⁺), while manganese (Mn) can show +2, +4, and +7. Non-transition metals like Sodium (Na) typically only shows a +1 oxidation state, and calcium (Ca) shows a +2 state. They have less flexibility because they don’t have d-electrons for bonding.
Question 14. Describe the preparation of potassium dichromate from iron chromite ore. What is the effect of increasing pH on a solution of potassium dichromate?
Answer: Preparation of Potassium Dichromate from Iron Chromite Ore:
Step 1: Fusion with Sodium Carbonate
Iron chromite ore (FeCr2O4) is mixed with sodium carbonate (Na2CO3) and heated in the presence of air. This oxidizes the chromite to form sodium chromate (Na2CrO4) and iron oxide (Fe2O3).
4FeCr2O4 + 8Na2CO3 + 7O2 → 8Na2CrO4 + 2Fe2O3 + 8CO2
Step 2: Formation of Sodium Dichromate
The sodium chromate formed is extracted with water and then acidified with sulfuric acid (H2SO4), converting it into sodium dichromate (Na2Cr2O7).
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Step 3: Conversion to Potassium Dichromate: Sodium dichromate is then treated with potassium chloride (KCl), resulting in the formation of potassium dichromate (K2Cr2O7), which is less soluble and crystallizes out.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
Effect of Increasing pH on Potassium Dichromate Solution:
At higher pH (in alkaline medium), potassium dichromate (K2Cr2O7) gets converted to chromate ions (CrO42-) which are yellow in color.
Cr2O72- + 2OH– → 2CrO42- + H2O
In acidic medium, the equilibrium shifts back, and dichromate ions (Cr2O72-), which are orange, dominate.
Question 15. Describe the oxidising action of potassium dichromate and write the ionic equations for its reaction with:
(i)iodide
(ii)iron (II) solution and
(iii)H2S
Answer: Oxidizing Action of Potassium Dichromate (K₂Cr₂O₇): Potassium dichromate is a powerful oxidizing agent, because it accepts electrons from other substances during reactions. In an acidic medium, it is reduced from Cr₂O₇²⁻ (dichromate ion) to Cr³⁺ (chromium ion), while the other substance is oxidized.
Ionic Equations:
1. With Iodide (I⁻): Potassium dichromate oxidizes iodide (I⁻) to iodine (I₂).
Cr₂O₇2- + 6I⁻ + 14H⁺ → 2Cr³⁺ + 3I₂ + 7H₂O
2. With Iron (II) (Fe²⁺) solution: Potassium dichromate oxidizes Fe²⁺ to Fe³⁺.
Cr₂O₇2- + 6Fe²⁺ + 14H⁺ → 2Cr³⁺ + 6Fe³⁺ + 7H₂O
3. With Hydrogen Sulfide (H₂S): Potassium dichromate oxidizes hydrogen sulfide to sulfur (S).
Cr₂O₇2- + 3H₂S + 8H⁺ → 2Cr³⁺ + 3S + 7H₂O
Question 16. Describe the preparation of Potassium permanganate. How does the acidified permanganate solution react with (a) iron (II) ions (b) SO2 (c) oxalic acid? Write the ionic equations for the reactions.
Answer: Preparation of Potassium Permanganate (KMnO₄): Potassium permanganate is prepared from manganese dioxide (MnO₂). Manganese dioxide is fused with potassium hydroxide (KOH) in the presence of air or an oxidizing agent like potassium nitrate (KNO₃). This forms potassium manganate (K₂MnO₄), which is then oxidized to potassium permanganate (KMnO₄) by passing chlorine or carbon dioxide through the solution.
Reactions of Acidified Potassium Permanganate:
1. With Iron (II) Ions (Fe²⁺):
MnO₄⁻ + 5Fe²⁺ + 8H⁺ → Mn²⁺ + 5Fe³⁺ + 4H₂O
Potassium permanganate oxidizes Fe²⁺ to Fe³⁺.
2. With Sulfur Dioxide (SO₂):
2MnO₄⁻ + 5SO₂ + 2H₂O → 2Mn²⁺ + 5SO₄²⁻ + 4H⁺
Potassium permanganate oxidizes SO₂ to sulfate ions (SO₄²⁻).
3. With Oxalic Acid (C₂H₂O₄):
2MnO₄⁻ + 5C₂O₄²⁻ + 16H⁺ → 2Mn²⁺ + 10CO₂ + 8H₂O
Potassium permanganate oxidizes oxalic acid to carbon dioxide (CO₂).
Question 17. For M2+/M and M3+/M2+ systems the E° values for some metals are as follows:
Cr2+/Cr = -0.9 V
Mn2+/Mn = -1.2V
Fe2+/Fe = -0.4 V
Cr3+/Cr2+ = -0.4 V
Mn3+/Mn2+ = + 1.5V
Fe3+/Fe2+ = + 0.8V
(i) The stability of Fe3+ in acid solution as compared to that of Cr3+ and Mn3+(ii)the ease with which iron can be oxidised as compared to a similar process for either chromium or manganese metal.
Answer: (i) Cr3+/Cr2+ has a negative reduction potential. Hence, Cr3+ cannot be reduced to Cr2+, that means Cr3+ is most stable. Mn3+/Mn2+ has large positive Eo value. Hence, Mn3+ can be easily reduced to Mn2+ that means Mn3+ is least stable. Eo value for Fe3+/Fe2+ is positive but small. Hence, Fe3+ is more stable than Mn3+ but less stable than Cr3+.
(ii) Oxidation potential for the given pairs will be + 0.9 V, + 1.2 V and +0.4 V. Thus, the order of their getting oxidised will be in the order: Mn > Cr > Fe.
Question 18. Predict which of the following will be coloured in aqueous solution?
Ti3+, V3+, Cu+, Sc3+, Mn2+, Fe3+, Co2+. and MnO4–.
Answer: Only those ions will be coloured which have incompletely filled d-orbitals. Those which have fully filled or empty d-orbitals are colourless. Hence, Ti3+, V3+, Mn2+, Fe3+and Co2+are coloured due to d-d transition. MnO4– is also coloured due to charge transfer. Only Sc3+ (3d0) is colourless.
Question 19. Compare the stability of +2 oxidation state for the elements of the first transition series.
Answer: The decreasing negative electrode potential of M2+/M in the first transition series shows that in general, the stability of +2 oxidation state decreases from left to right except Mn and Zn. The decrease in the negative electrode potential is due to increase in the sum of IE1 + IE2. The greater stability of Mn is due to half filled d-subshell (d5) in Mn2+ and that of Zn is due to completely filled of d-subshell (d10) in Zn2+.
Question 20. Compare the chemistry of actinoids with that of the lanthanoids with special reference to
(i)electronic configuration,
(ii)atomic and ionic sizes and
(iii)oxidation state
(iv)chemical reactivity.
Answer: Comparison of Actinoids and Lanthanoids:
(i) Electronic Configuration:
Both actinoids and lanthanoids have their f-orbitals progressively filled. Lanthanoids fill 4f orbitals, while actinoids fill 5f orbitals. Actinoids have more complex configurations due to the involvement of 5f, 6d, and 7s orbitals.
(ii) Atomic and Ionic Sizes:
Both groups show a steady decrease in atomic and ionic sizes across the series, called lanthanoid and actinoid contraction. However, actinoid contraction is less regular than lanthanoids.
(iii) Oxidation States:
Lanthanoids mainly exhibit a +3 oxidation state, while actinoids show a wider range of oxidation states (+3, +4, +5, +6) due to the more variable participation of 5f electrons.
(iv) Chemical Reactivity:
Actinoids are generally more reactive than lanthanoids. They react easily with air, water, and acids. Actinoids are also more electropositive and have a higher tendency to form complexes.
Question 21. How would you account for the following:
(i) Of the d4 species, Cr2+ is strongly reducing while manganese (III) is strongly oxidizing.
(ii) Cobalt (II) is stable in aqueous solution but in the presence of complexing reagents it is easily oxidised.
(iii) The d1 configuration is very unstable in ions.
Answer: (i) Cr²⁺ is strongly reducing because it easily loses an electron to form Cr³⁺, which has a more stable electronic configuration. In contrast, Mn³⁺ is strongly oxidizing because it tends to gain an electron to form Mn²⁺, which is more stable.
(ii) Cobalt(II) is stable in water, but when complexing agents are present, they stabilize the Co³⁺ ion, making the oxidation of Co²⁺ to Co³⁺ easier.
(iii) d¹ configuration is unstable because ions with a single electron in the d-orbital tend to lose that electron, leading to a more stable configuration with empty or more filled d-orbitals.
Question 22. What is meant by disproportionation? Give two examples of disproportionation reaction in aqueous solution.
Answer: Disproportionation is a type of redox reaction where the same element is both oxidized and reduced, forming two different products with different oxidation states.
Examples: 1. Chlorine in water:
Cl2 + H2O → HCl + HOCl
Chlorine is reduced to HCl (Cl⁻) and oxidized to HOCl (Cl⁺).
2. Manganese in basic solution:
2MnO4– + H2O → MnO2 + MnO42- + 2OH–
Manganese is reduced to MnO₂ (Mn⁴⁺) and oxidized to MnO42- (Mn⁶⁺).
Question 23. Which metal in the first transition series exhibits +1 oxidation state most frequently and why?
Answer: Cu has the electronic configuration 3d10 4s1. It can easily lose 4s1 electron to give the stable 3d10 configuration. Hence, Cu shows +1 oxidation state.
Question 24. Calculate the number of unpaired electrons in the following gaseous ions: Mn3+, Cr3+, V3+ and Ti3+. Which one of these is most stable in aqueous solution?
Answer: In Mn3+ = 3d4 = 4 unpaired electrons. Cr3+ = 3d3 = 3 unpaired electrons. V3+ = 3d2 = 2 unpaired electrons. Ti3+ = 3d1 = 1 unpaired electron. Cr3+ is more stable out of them in aqueous solution because its half-filled t₂g configuration (3d³), which provides extra stability.
Question 25. Give examples and suggest reasons for the following features of the transition metal chemistry:
(i) The lowest oxide of transition metal is basic the highest is amphoteric/ acidic.
(ii) A transition metal exhibits highest oxidation state in oxides and fluorides.
(iii) The highest oxidation state is exhibited in oxoanions of a metal.
Answer: (i) Lowest oxide is basic, highest is amphoteric/acidic because in lower oxidation states, the metal has more metallic character, making the oxide basic. As oxidation state increases, the metal behaves more like a non-metal, making the oxide acidic or amphoteric. For example Example: MnO (basic), Mn₂O₇ (acidic)
(ii) A transition metal exhibits highest oxidation state in oxides and fluorides because oxygen and fluorine are highly electronegative, pulling electrons from the metal, which stabilizes the metal in its highest oxidation state. For example Mn₂O₇, VF₅.
(iii) Highest oxidation state is exhibited in oxoanions because the presence of oxygen atoms in oxoanions stabilizes the high oxidation state of the metal by delocalizing the charge over the oxygen atoms. For example Cr₂O₇²⁻ (chromate ion).
Question 26. Indicate the steps in the preparation of:
(i)K2Cr207from chromite ore
(ii)KMn04 from pyrolusite ore.
Answer: (i) Preparation of K₂Cr₂O₇ from chromite ore:
1. Roasting: Chromite ore (FeCr₂O₄) is roasted with sodium carbonate (Na₂CO₃) and oxygen to form sodium chromate (Na₂CrO₄).
4FeCr₂O₄ + 8Na₂CO₃ + 7O₂ → 8Na₂CrO₄ + 2Fe₂O₃ + 8CO₂
2. Conversion: Sodium chromate is treated with sulfuric acid (H₂SO₄) to form sodium dichromate (Na₂Cr₂O₇).
2Na₂CrO₄ + H₂SO₄ → Na₂Cr₂O₇ + Na₂SO₄ + H₂O
3. Purification: Sodium dichromate is treated with potassium chloride (KCl) to form potassium dichromate (K₂Cr₂O₇).
Na₂Cr₂O₇ + 2KCl → K₂Cr₂O₇ + 2NaCl
(ii) Preparation of KMnO₄ from pyrolusite ore:
1. Oxidation: Pyrolusite (MnO₂) is fused with potassium hydroxide (KOH) and heated with oxygen to form potassium manganate (K₂MnO₄).
2MnO₂ + 4KOH + O₂ → 2K₂MnO₄ + 2H₂O
2. Disproportionation: Potassium manganate is oxidized in an acidic medium to form potassium permanganate (KMnO₄).
3K₂MnO₄ + 2H₂O → 2KMnO₄ + MnO₂ + 4KOH.
Question 27. What are alloys? Name an alloy which contains some lanthanoid metals. Mention its uses.
Answer: An alloy is a homogeneous mixture of two or more metals or metals and nonmetals. An important alloy containing lanthanoid metals is misch metal which contains 95% lanthanoid metals and 5% iron along with traces of C, S, Cu and Al. It is used in Mg-based alloy to produced bullets, shells and flints.
Question 28. What are inner transition elements? Decide which of the following atomic numbers are the atomic numbers of the inner transition elements: 29,59,74,95,102,104.
Answer: The f-block elements are those in which the last electron enters into f-subshell are called inner transition elements. These include lanthanoids (58 – 71) and actinoids (90 – 103). Thus, elements with atomic numbers 59, 95 and 102 are inner transition elements.
Question 29. The chemistry of the actinoid elements is not so smooth as that of the lanthanoids. Justify this statement by giving some examples from the oxidation state of these elements.
Answer: Lanthanoids show limited number of oxidation states. They are +2, +3 and +4. Out of which +3 is common. This is because of large energy gap between 4f, 5d and 6s subshells. The dominant oxidation state of actinoids is also +3 but they show a number of other oxidation states also. For example uranium (Z = 92) and plutonium (Z = 94) show +3, +4, +5 and +6. This is due to small energy difference between 5f, 6d and 7s subshells of actinoids.
Question 30. Which is the last element in the series of the actinoids? Write the electronic configuration of this element. Comment on the possible oxidation state of this element.
Answer: Last actinoid = Lawrencium (Z = 103). Electronic configuration = [Rn]86 5f14 6d1 7s2. Possible oxidation state = +3.
Question 31. Use Hund’s rule to derive the electronic configuration of Ce3+ ion and calculate its magnetic moment on the basis of spin only formula.
Answer: 58Ce = [Xe]54 4f15d16s2 and Ce3+ = [Xe]54 4f1 that means there is only one unpaired electron and hence, n = 1. Now μ (magnetic moment) = √n(n + 2 = √1(1 + 2 = √3 = 1.73 BM.
Question 32. Name the members of the lanthanoid series which exhibit +4 oxidation states and those which exhibit +2 oxidation states. Try to correlate this type of behaviour with the electronic configuration of these elements.
Answer: +4 = 58Ce, 59Pr, 60Nd, 65Tb, 66Dy. +2 = 60Nd, 62Sm, 63Eu, 69Tm, 70Yb. +2 oxidation state is exhibited when the lanthanoid has the configuration 5d0 6s2 so that 2 electrons are easily lost. +4 oxidation state is exhibited when the configuration left is close to 4f0, 4f1, 4f2, 4f7, 4f8.
Question 33. Compare the chemistry of actinoids with that of lanthanoids with reference to (i) electronic configuration (ii) oxidation states and (iii) chemical reactivity.
Answer: (i) Electronic Configuration:
Lanthanoids: The electrons are added to the 4f orbitals. Their general configuration is [Xe] 4f¹⁻¹⁴ 5d⁰⁻¹ 6s².
Actinoids: The electrons are added to the 5f orbitals. Their general configuration is [Rn] 5f¹⁻¹⁴ 6d⁰⁻² 7s².
(ii) Oxidation States:
Lanthanoids: They mostly exhibit a +3 oxidation state, though +2 and +4 are also possible in a few elements.
Actinoids: They show a wider range of oxidation states, from +3 to +6 or higher, because of the greater involvement of 5f electrons.
(iii) Chemical Reactivity:
Lanthanoids: They are less reactive compared to actinoids and typically react slowly with air and water.
Actinoids: They are more reactive, especially in the early actinoids, and react more readily with air, water, and acids due to their larger atomic size and more available electrons.
Question 34. Write the electronic configuration of the elements with atomic numbers 61, 91, 101 and 109.
Answer: Z=61 (Promethium, Pm) = [Xe]544f55d0 6s2
Z = 91 (Protactinium, Pa) = [Rn]86 5f2 6d1 7s2
Z = 101 (Mendelevium, Md)= [Rn]86 5f13 6d0 7s2
Z = 109 (Meitnerium, Mt) [Rn]86 5f14 6d7 7s2
Question 35. Compare the general characteristics of the first series of the transition metals with those of the second and third series metals in the respective vertical columns. Give special emphasis on the following points:
(i)electronic configurations
(ii)oxidation states
(iii)ionisation enthalpies and
(iv)atomic sizes
Answer: The transition metals have been divided into three series: first, second, and third. The comparison of their general characteristics with respect to the given points are followings:
(i) Electronic configurations:
- The first series (Sc to Cu) has electrons filling the 3d orbitals.
- The second series (Y to Ag) fills 4d orbitals, and the third series (La to Au) fills 5d orbitals.
- The overall pattern of electron filling is similar, but the higher series have more inner d-electrons.
(ii) Oxidation states: All three series show variable oxidation states, but the higher series (second and third) tend to show higher oxidation states more frequently. For example, tungsten (W) and osmium (Os) show +6 and +8 oxidation states, respectively, which are not common in the first series.
(iii) Ionisation enthalpies: Ionisation enthalpies increase down the group, meaning that the second and third series elements have higher ionisation energies than the first series. However, due to the lanthanide contraction, the third series has ionisation enthalpies similar to the second series.
(iv) Atomic sizes: Atomic sizes increase from the first to the second series, but there is only a small difference between the second and third series. This is due to the lanthanide contraction, which causes the atoms in the third series to be nearly the same size as those in the second series.
Question 36. Write down the number of 3d electrons in each of the following ions:Ti2+, V2+, Cr3+, Mn2+, Fe2+, Fe2+, Co2+, Ni2+ and Cu2+. Indicate how would you expect the five 3d orbitals to be occupied for these hydrated ions (octahedral).
Answer: 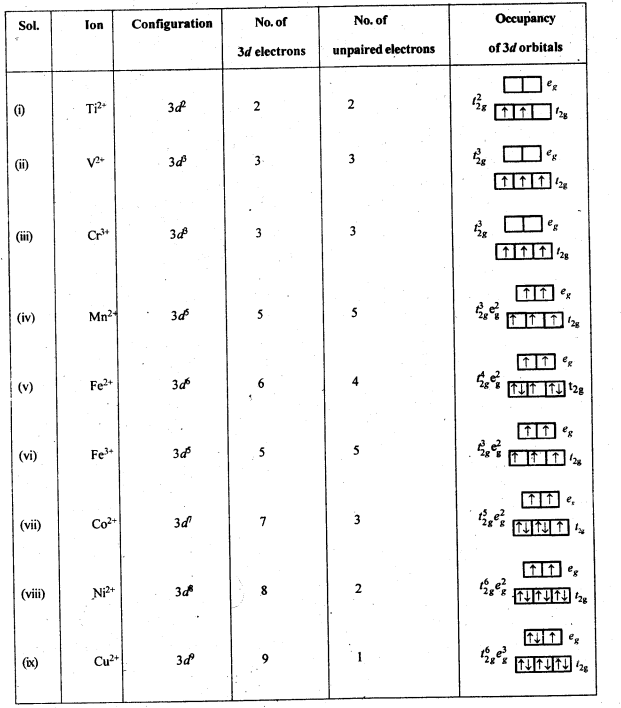
Question 37. Comment on the statement that elements of first transition series possess many properties different from those of the heavier transition elements.
Answer: The elements of the first transition series (3d series) have several properties different from the heavier transition elements (4d and 5d series) due to their smaller size and lower atomic mass. Some key differences are followings:
1. Atomic and ionic sizes: The elements of the 3d series are smaller compared to 4d and 5d elements, which affects their bonding and reactivity.
2. Ionization energy: The first transition series generally has lower ionization energies, making them more reactive.
3. Complex formation: 4d and 5d series elements tend to form more stable complexes due to their larger size and higher availability of d-orbitals.
4. Oxidation states: Heavier transition elements often show a wider range of oxidation states.
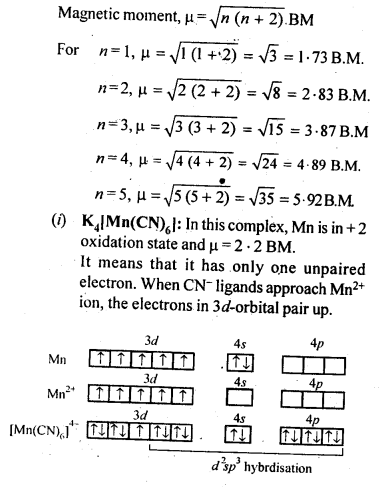
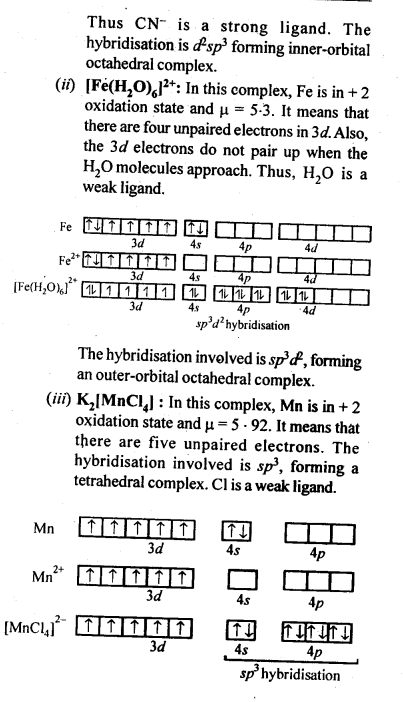
Question 38. What can be inferred from the magnetic moment values of the following complex species? 
Answer: