As we know that transformation of matter in chemistry involves various types of reactions. One important category of these reactions is Redox Reactions. A number of phenomena either physical or biological are concerned with redox reactions. The importance of these reactions can be seen in burning of different types of fuels for obtaining energy. These energy are useful for domestic, transport and other commercial purposes.
Electrochemical processes like extraction of highly reactive metals and nonmetals, preparation of chemical compounds like NaOH, operation of dry and wet batteries and corrosion of metals are also studied under redox processes. Of late Hydrogen Economy and development of Ozone Hole have been figuring under redox reactions. Here we shall learn all the important topics which are necessary for preparation of entrance and competitive exams.
Classical concepts about Redox Reactions
Oxidation. The reaction in which addition of oxygen/electronegative element to a substance or removal of hydrogen/electropositive element from a substance take place is called oxidation. For example.
2Mg(s) + O2 (g) → 2MgO(s)
2Mg(s) + Cl2 (g) → MgCl2(s)
2H2S(g) + O2 (g) → 2S(s) + H2O (l)
2KI(aq) + H2O (l) + O3(g) → 2KOH (aq) + I2(s) + O2 (g)
Reduction. The reaction in which addition of hydrogen/electropositive element to a substance or removal of oxygen/electronegative element from a substance take place is called reduction. For example.
Br2(g) + H2S(g) → 2HBr(g) + S(s)
2HgCl2(aq) + SnCl2(aq) → Hg2Cl2(s) + SnCl4(aq)
CuO(s) + H2(g) → Cu(s) + H2O (l)
2FeCl3 (aq) + SO2(g) + 2H2O (l) → 2FeCl2(aq) + H2SO4 (aq) + 2HCl
Redox Reaction
The chemical reactions in which oxidation and reduction take place simultaneously are called redox reactions. For example.
H2S(g) + Cl2(g) → 2HCl(g) + S(s)
Here, H2S is oxidised to S and Cl2 is reduced to HCl.
Redox Reactions In Terms Of Electron Transfer Reactions
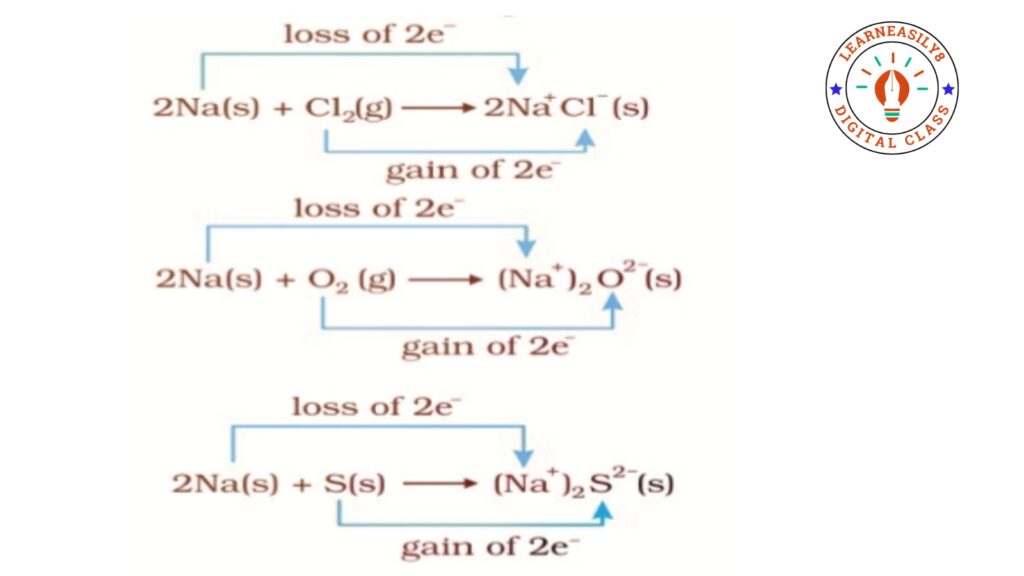
Oxidation. A chemical process in which electrons are lost is called oxidation. The chemical substance which are involved in losing of electrons are called Reducing Agent.
Reduction. A chemical process in which electrons are gained is called Reduction. The chemical substance which are involved in losing of electrons are called Oxidising Agent. Examples are followings:-
Oxidation Number
The charge assigned on the atom of any element when present in a molecule or ion is called Oxidation number. For example
The oxidation number of Hydrogen in H2, HCl and NaH are 0, +1 and -1 respectively.
Rules for assigning Oxidation Numbers in Redox Reactions
The following rules are applied to determine the oxidation number of an atom in an ion or a molecule.
1. The oxidation number of all the atoms of different elements in their respective elementary states and allotropic forms is taken as zero. For example, the oxidation number of each atom in N2, Cl2, He, P4, O3, C(diamond) is zero.
2. The oxidation number of a mono- atomic ion is same as the charge on it. For example, oxidation number of Na+, Mg2+, Cl–, O2- are +1, +2, -1 and -2 are respectively.
3. The oxidation number of hydrogen is +1 when combined with non-metals and is -1 when linked with active metals. For example, oxidation number of H in HCl and LiH are +1 and -1 respectively.
4. The oxidation number of oxygen is -2 in most of the compounds, except in peroxide and fluoride. For example, the oxidation number of oxygen in H2O2, O2F2 and OF2 are -1, +1 and +2 respectively.
5. In binary compounds of metals and nonmetals, metal atoms will have positive oxidation number and nonmetals will have negative oxidation number equal to their valency. For example, the oxidation number of K and Cl in KCl are +1 and -1 respectively.
6. The oxidation number of the alkali metals and alkaline earth metals in combined states are +1 and +2 respectively.
7. The oxidation number of F and Al are also -1 and +3 always in combined state.
8. In neutral compounds, the sum of the oxidation numbers of all the atoms is zero. In complex ions, the sum of all the atoms in the ion is equal to the charge on the ion. For example, in K2Cr2O7, the sum of the oxidation number of K(+2×1) + Cr(+6×2) + O(-2×7) = 0.
Read More: Ionic Equilibrium: short and easy notes for class 11th
The Paradox of Fractional Oxidation States
In many compounds, the oxidation number of a particular element is either fractional or greater than the group oxidation state. This oxidation number is known as pardoxial oxidation number. For example the oxidation number of C in C3O2 (carbon suboxide) is 4/3 that of Br in Br3O8 (tribromooctaoxide) is 16/3 and that of S in Na2S4O6 (Sodium tetrathionate) is 2.5.
Since electrons are never transferred or shared in fraction, therefore, the very idea of fractional oxidation states is unconvincing. In fact, fractional oxidation state is only the average oxidation state of an element when two or more of its atoms are present in different oxidation states in a given compound. Examples are followings:-
Oxidation Numbers and Nomenclature
The compounds of metals which show more than one oxidation states are distinguished from one another by placing a Roman numeral such as I, II, III, IV etc. indicating the oxidation state of the metal within parenthesis after the symbol or name of the metal. For example, Copper forms two oxides, these two oxides are distinguished as Cu2(I)O and Cu(II)O. This system of nomenclature was introduced by Alfred Stock, a German chemist, and is commonly known as Stock notation after his name.
Some other examples are followings:-
FeSO4, — Fe(ll)SO4
Fe2(SO4)3 — Fe2(lll)(SO4)3
Cr2O3 — Cr2(lll)O3
K2Cr2O7—— K2Cr2(Vl)O7
KMnO4 — KMn(Vll)O4.
Types of Redox Reactions
1. Combination reactions. A reaction in which two atoms or molecules combine together to form a third molecule is called a combination reaction. For example,
A + B → C
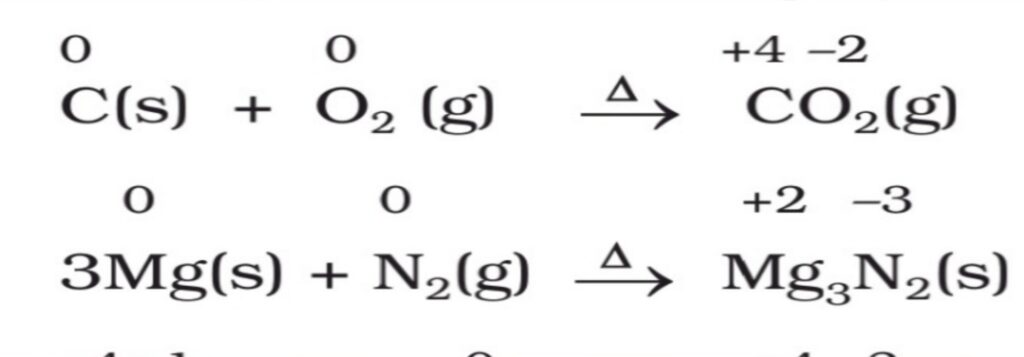
2. Decomposition reactions. A reaction in which a molecule breaks down to form two or more chemical components is called a decomposition reaction. For example,
AB → A + B
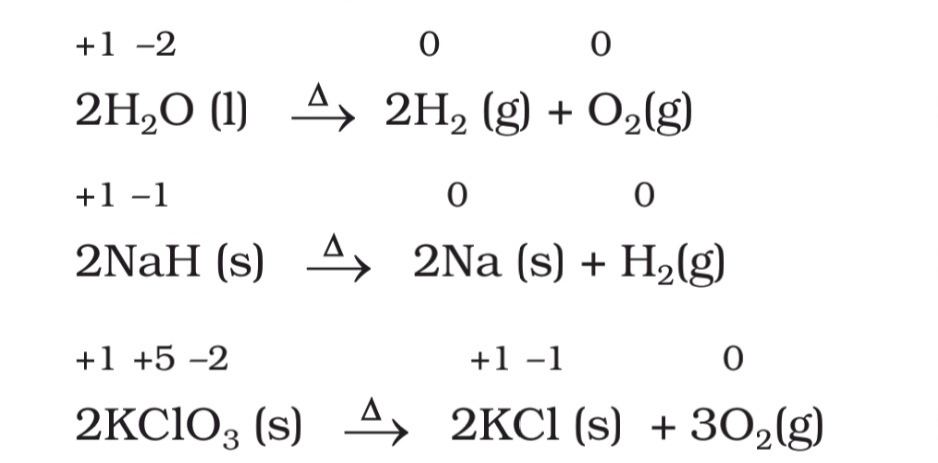
3. Displacement reactions. A reaction in which an atom or ion in a compound is replaced by an atom or ion of some other element, is called a displacement reaction. In general, it is represented by the equation, X + YZ → XZ + Y.
Types of displacement reactions. Displacement reactions are of two types:
(a) Metal- displacement reactions
(b) Non- metal displacement reactions.
(a) Metal- displacement reactions. In these reactions, a metal in the compound is displaced by some other metal in the elemental state. For example,
(b) Non- metal displacement reactions. In these reactions, a metal or a non- metal in the compound is displaced by some non- metal in the elemental state. For example,
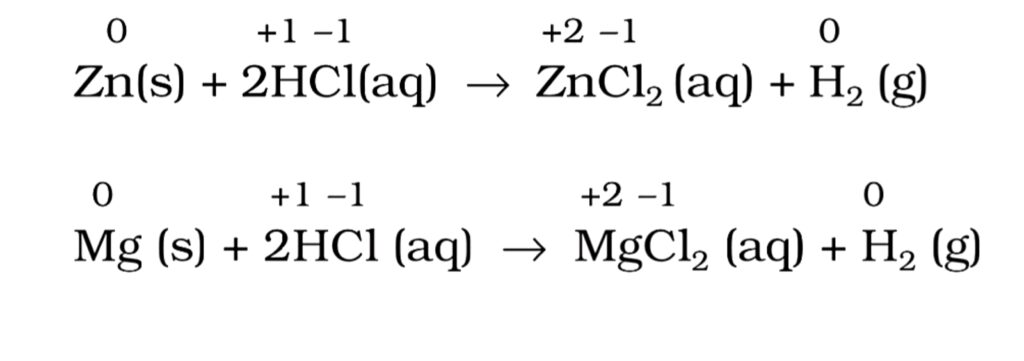
4. Disproportionation reactions. A reaction in which the same species is simultaneously oxidised as well as reduced is called a disproportionation reaction. For such reactions to occur, the reducing species must have an element which has at least three oxidation states. For example
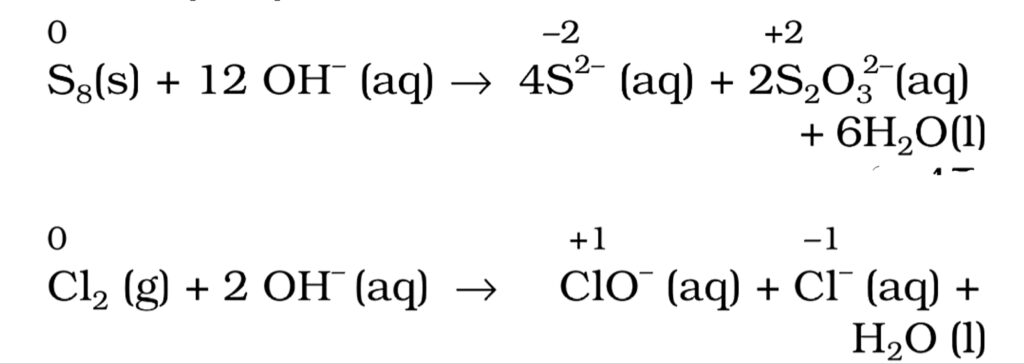
Redox Reactions based on Oxidation Number
Oxidation. A chemical change in which oxidation number of any element increases is called oxidation.
Reduction. A chemical change in which oxidation number of any element decreases is called reduction.
Oxidising agent. A chemical substance which involves in reduction is called oxidising agent.
Reducing agent. A chemical substance which involves in oxidation is called reducing agent.
For example:
Balancing of Redox Reactions
A chemical equation which involves in oxidation and reduction can be balanced with the help of the following two methods:
1. Oxidation number method
2. Ion electron method or half equation method
Oxidation Number Method
The following steps involved in the balancing of Redox Reactions
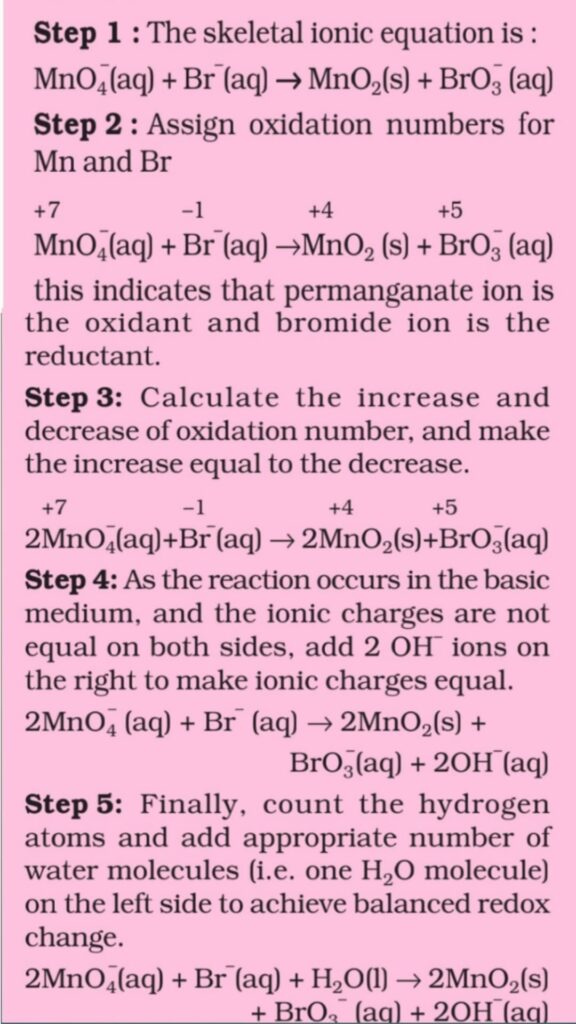
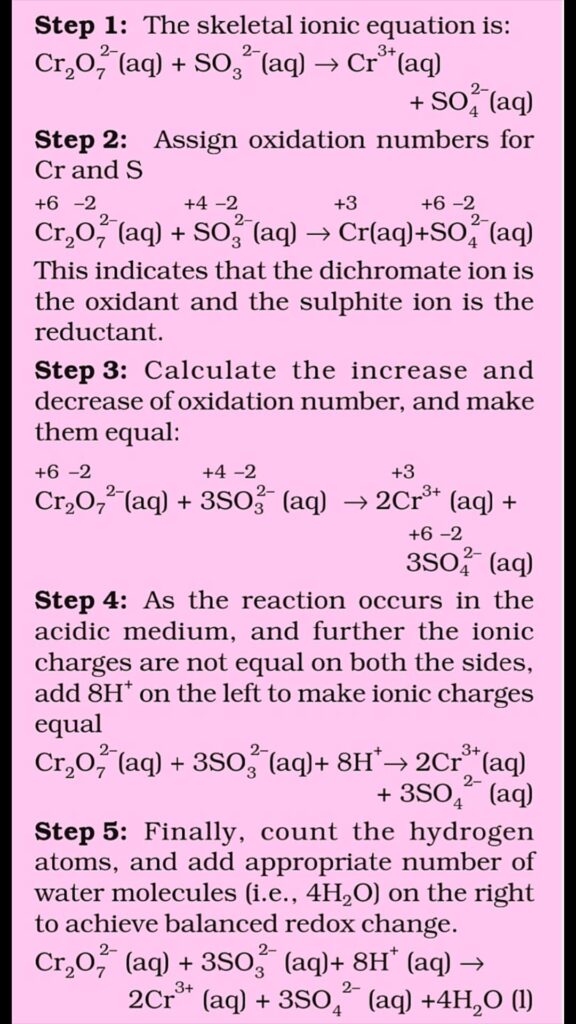
Step 1. Write the skeletal equation of of all the reactants and products of the equation.
Step 2. Indicate the oxidation number of each element above its symbol and identify the elements which undergo a change in the oxidation number.
Step 3. Calculate the increase or decrease in oxidation number per atom and identify the oxidising and reducing agents. If more than one atom of the same element is involved, find out the total increase or decrease in O.N. by multiplying this increase or decrease in O.N. per atom by the number of atom by undergoing that change.
Step 4. Multiplying the formulae of the oxidising and the reducing agents by suitable integers so as to equalize the total increase or decrease in oxidation number as calculated in the above step.
Step 5. Balance all atoms other than H and O by Hit and Trial method.
Step 6. Finally balance H and O atoms by adding H2O molecules using hit and trial methods.
Step 7. In case of Ionic reactions,
(a) For acidic medium. First balance O atoms by adding H2O molecules to whatever side deficient in O atoms and then balance H atoms by adding H+ ions to whatever Side deficient in H atoms.
(b) For basic medium. First balance O atoms by adding H2O molecules to whatever Side deficient in O atoms. The atoms are then balanced by adding H2O molecules in number to the deficiency of H atoms and an equal number of OH– ions are added to the opposite side of the equation. Now remove the duplication if any. For example: In acidic medium and in basic medium respectively
Half equation method
This method of balancing Redox equations is based upon the principle that electrons lost during oxidation half reaction of any Redox Reaction are equal to the electrons gained during reduction half reaction. The following steps are involved in this method:
Step 1. Write the skeletal equation and indicate the oxidation number of all the elements which appear in the skeletal equation above their respective symbols.
Step 2. Find out the species which are oxidised and reduced.
Step 3. Split the skeletal equation into two half reactions as oxidation half reaction and reduction half reaction
Step 4. Balance the two half reaction equation by the following rules:
(l) In each half reaction, first balance the atoms of the elements which have undergone a change in oxidation number.
(ll) Add electrons to whatever Side is necessary to make up the difference in oxidation number in each half reaction.
(lll) Balance charge by adding H+ ions if the reaction occurs in the acidic medium and by adding OH– ions if the reaction occurs in basic medium.
(lV) Balance oxygen atoms by adding required number of H2O molecules to the side deficient in O atoms.
(V) In the acidic medium, H atoms are balanced by adding H+ ions to the Side deficient in H atoms. However in the basic medium, H atoms are balanced by adding H2O molecules equal in number to the deficiency of H atoms and an equal number OH– ions are included in the opposite side of the equation. Now remove the duplication, if any.
Step 5. The two half reactions are then multiplied by suitable integers so that the total number of electrons gained in one half reaction is equal to the number of electrons lost in the other half reaction. The two half reactions are then added up.
Step 6. Now verify that whether the equation is balanced or not, the total charge on the either side of the equation must be equal.
Redox Titration in Redox Reactions
The determination of the exact amount of an oxidising agent in a given solution by titrating it against the standard solution of a suitable reducing agent or vice versa is called redox titration.
Types of redox titration
Depending upon the nature of the oxidising agent, there are four types of redox titration:
1. Potassium permanganate titration. In this titration, reducing agents like FeSO4, Mohr’salt, Oxalic acid, oxalates etc. are directly titrated against KMnO4 as the oxidising agent in acidic medium.
Here KMnO4 acts as the self indicator. In this titration, when whole of the reductant is oxidised, end point is reached. At the end point, the violet colour of MnO4– solution disappears and a lasting tinge of pink colour appears. This colour change is so sensitive that it can be easily detected even at very low concentration. Reaction is as
5Fe2+ + MnO4– + 8H+ → 5Fe3+ + Mn2+ + 4H2O
2. Potassium dichromate titration
In this titration, the above listed reducing agents are directly titrated against K2Cr2O7, as the oxidising agent in acidic medium.
Here, diphenylamine is used as an indicator which produces intense blue colour at the end point. Reaction is as:
6Fe2+ + Cr2O72- + 14H+ → 2Cr3+ 6Fe3+ + 7H2O
3. Iodimetric titration. This titration involve the direct use of iodine as the oxidising agent in neutral or slightly acidic medium using starch as an indicator. The various reducing agents used in these titration are thiosulphate, sulphites, arsenite and antimonite. Reactions are as:
I2 + 2S2O32- → 2I– + S4O62-
I2 + SO32- + H2O → 2I– + SO42- + 2H+
I2 + AsO33- + H2O → 2I– + AsO43- + 2H+
I2 + SbO33- + H2O → 2I– + SbO43- + 2H+
4. Iodometric titration. This titration is carried out in two steps. In the first step, oxidising agents such as KMnO4, K2Cr2O7, CuSO4, peroxides etc. are treated with an excess of KI when I2 is liberated quickly and quantitatively. For example,
2MnO4– + 16H+ + 10I– → 2Mn2+ + 5I2 + 8H2O
Cr2O72- + 14H+ +6I– → 2Cr3+ 3I2 + 7H2O
In the second step, the liberated Iodine is titrated against a standard solution of Sodium thiosulphate using starch as indicator.
Mathematical formula for Redox Titration:
M1V1/n1 = M2V2/n2
Where M1 and M2 are the molarities of the reductant and oxidant or vice versa and V1 and V2 are their Volumes and n1 and n2 represent their stoichiometric coefficients.
Conclusion: Redox Reaction
Redox Reactions form an important class in which oxidation and reduction take place simultaneously. In this article, the definition of oxidation, reduction, oxidising agent and reducing agent have been defined clearly on classical, electronic and oxidation concept. Determination of oxidation number and balancing of Redox Reactions on oxidation number and ion-electron method have been explained with a consistent set of rules. the classification of Redox Reactions have been detailed in proper manner with suitable examples.
FAQ in Redox Reactions
Q.NO. 1. What is a redox couple?
A redox couple consists of oxidised and reduced form of the same substance taking part in an oxidation or reduction half reaction.
Q. NO. 2. What do you mean by EMF of the cell?
EMF of a cell is defined as the difference in the difference in the electrode potentials of the two half cells when the cell is not sending current through the circuit.
Q. NO. 3. What is an electrode potential?
The tendency of an electrode to lose or gain electrons is called electrode potential.
Q. NO. 4. What is an electrochemical cell in Redox Reaction?
A device that is used to convert chemical energy in an indirect redox reaction into chemical energy is called an electrochemical cell.
Q.NO. 5. What do you mean by Electrochemical series?
A list of redox couple arranged in decreasing order of standard reduction potential is called electrochemical series.