Solution and Its Type: Solution is an impure substance. It contains varying amounts of the components. In our everyday life, we rarely come across pure substances. Hence, solution is important in our daily life. We shall learn in detail about the types of solution and its importance in our life.
What is called solution?
As we know that solution is a homogeneous mixture in which composition and properties are uniform throughout the mixture. It can be defined in the following way:
“A solution is a homogeneous mixture of two or more chemically non- reacting substances whose composition can be varied within certain limit”.
Some examples of solution: Alloys, air, hydrated salts, aerated drinks, alcohol in water.
Solution and Its Type
Types of components in a solution: There are two components in any solution.
- Solvent: The chemical substance which is maximum in any solution is called solvent.
- Solute: The chemical substances which are present in a solution other than solvent are known as solute.
- Example: If a solution contains 50 g of A,10 g of B and 20 g of C then A will be called solvent. B and C will be known as solutes.
Binary solution: When a solution contains only two chemical substances in the form of homogeneous mixture. It is called binary solution. For example a solution of sugar and water.
Types of solution based on solvent:
Based on the solvent, there are two types of solution.
1. Aqueous solution: When solvent is water in a solution, it is called binary solution. For example a solution of sugar and water.
2. Non aqueous solution: When solvent is other than water in any solution, it is called non- aqueous solution. For example a solution of benzene and toluene.
Types of solution based on the physical state of components:
There are nine types of solutions based on the physical state of solutes and solvent. 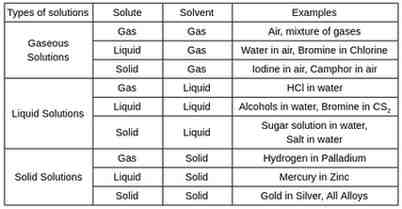
Classification of solution: Based on osmotic pressure
There are three types of solutions based on osmotic pressure.
1. Isotonic solution. The solutions which have same osmotic pressure at the same temperature are called isotonic solutions. For example, A 0.9 % solution of pure NaCl is isotonic with human red blood cells (RBC). In the presence of this solution, RBC neither swell nor shrink.
2. Hypotonic solution. A solution which has the lowest osmotic pressure among others at the same temperature is called hypotonic solution. For example, a pure NaCl solution with concentration less than 0.9 % is called hypotonic solution. On placing RBC in this solution, they will swell and even burst.
3. Hypertonic solution. A solution which has the highest osmotic pressure among others at the same temperature is called hypertonic solution. For example, a pure NaCl solution with concentration more than 0.9 % is called hypertonic solution. On placing RBC in this solution, they will shrink due to plasmolysis.
Solutions of solids. Such a type of solution in which the solvent is solid called solutions of solids. But the common examples of them are are those in which both solute and solvent are solid. For example alloys. These solids are classified into two types as follows:
1. Substitutional solid solution. If some atoms, ions or molecules of one solid present at the lattice sites are replaced by the atoms, ions or molecules of another similar solid, having similar sizes, the solids thus obtained are called substitutional solid solution. For example, brass, bronze, steel etc.
2. Interstitial solid solution. If in the lattice of a solid, the atoms of some other solid, small in size, occupy the interstitial sites or voids. The solids obtained are called interstitial solid solution. For example tungsten carbide.
Classification of solution based on the amount of solutes in liquid solvent.
There are three types of solutions.
1. Saturated solution. Such a solution in which no more solutes can be dissolved at the certain temperature is called saturated solution.
2. Hyposaturated solution. A solution in which more solutes can be dissolved is called hyposaturated solution.
3. Hypersaturated solution. A solution which has more solutes than its solubility at the normal temperature is called hypersaturated solution.
Read More : Notes of solution for chemistry 12th chapter two
Solution and Its Type: Importance of solution
The use of a particular solution is very useful in our daily life. Their uses depend on the composition of solutions.
1. Brass is a homogeneous mixture of copper and zinc. German silver is that of copper, zinc and nickel while bronze is that of copper and tin. They have different properties and hence are put to different uses.
2. 1 part per million parts (1ppm) of fluoride ions in water prevents tooth decay. 1.5 ppm of fluoride causes the teeth to become mottled and still higher concentration can be used as poison.
3. Solutions of intravenous injections should have the same ionic concentration as that of the blood plasma.
Read Also: Colligative Properties: Classification, Definition, Formula and Application for 12th
Solution and Its Type: Conclusion
In this notes, (Solution and Its Type) we have tried our best to explain the important topics of this chapter in simple and easy manners. We hope that you must have liked this notes and if so then share among your friends and favourites. You can also take help Self study.com.
