You are welcome to visit this website. We shall learn about structure of atoms and important models of atomic structure. This chapter is very important for all the students who are preparing for jee mains, IIT, neet and other competitive exams. This chapter also help us to understand other chapters and topics of chemistry. We have tried our best to explain every concepts very clearly and simply. Let’s we start with.
Structure of atoms in simple ways:
Structure of atoms needs to know about the shape of the atoms and what contents are present inside it. We shall discuss the simple structure and important terms to be applied to understand the important terms during study of the physical and chemical properties of the atoms of particular elements.
What is an atom?
An atom is the smallest part of an element which has no existence but takes part in a chemical reaction. For example H is an atom of hydrogen. O is an atom of oxygen.
Structure of an atom.
An atom of any element is spherical in shape. Any atom consists of mainly three types of particles and they are known as fundamental particles. They are protons, neutrons and electrons. protons and neutrons are present in the nucleus of an atom and they are known as nucleons. Electrons move around the nucleus in various circular paths and these paths are called orbits.
Discovery of Fundamental particles
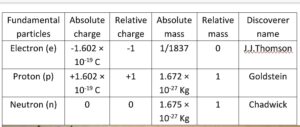
Atomic number and Mass number
Atomic number. Total number of protons present in the nucleus of the atom of any element is called atomic number. It is denoted by “Z”.
Z = p . Where p = total no. Of electrons.
Mass number. The sum of the protons and neutrons present in the nucleus of the atom of any element is called mass number. It is denoted by “A”, A = p + n or A = z + n.
Different types of atomic species
Isotopes. Those elements which have same atomic number but different mass number are known as isotopes. Ex:- 1H1, 1H2 and 1H3.
Isobars. Those elements which have same mass numbers but different atomic numbers are called isobars. Ex:- 19K40, 20Ca40.
Isotones. Those elements which have same number of neutrons are called isotones. Ex:- 14Si30, 15P31.
Isoelectronic. Those species which have same number of electrons are called isoelectronic. Ex:- Na+, Mg2+, Al3+, Ne, N3-, O2-.
Isodiapheres. Atoms having the same difference in neutrons and protons as n – Z are called isodiapheres. Ex. 19K39, 9F19.
Important models of atomic structure
1.Thomson’ Model
J.J.Thomson proposed that an atom possesses a spherical shape (radius 10-10m) in which the positive charge is uniformly distributed. The electrons are embedded into it in such a manner as to give the most stable electrostatic arrangement. This model can be visualised as a pudding, or watermelon.
Rutherford Model of Atomic Structure:
1. Most of the mass and whole of positive charge of an atom is centered in a very small region called the nucleus.
2. Major portion of the atom is empty space and the magnitude of the positive charge is different for different atoms and is nearly one half of the atomic weight of the element.
3. Since the atom as a whole is electrically neutral so there exists as many electrons outside the nucleus as there are units of positive charge in the nucleus.
4. Electrons remove around the nucleus in closed orbits with high speed. The centrifugal force acting on the revolving electron is counter balanced by the force of attraction between the nucleus and the electrons.
5. As this model was closed to the model of planets going around sun, hence it was called planetary model.
Limitations of Rutherford Model
1. Rutherford model could not explain about the energy that is required for continuous moving electrons around the nucleus.
2. This model could not explain any of the properties of atom including their line spectra.
Bohr’s Model of Atomic Structure
1. An atom consists of positively charged nucleus responsible for almost the entire mass of atom.
2. The electrons in an atom revolve around the nucleus only in certain fixed concentric, circular orbits called shells.
3. Each orbit or shell is associated with a definite amount of energy. So these are now called energy levels.
4. The energy of these shells goes on increasing as the distance from the nucleus increases. However the difference in the energy of the successive shells goes on decreasing.
5. As long as the electron revolves in a particular orbit or shell, it neither absorbs nor emits energy continuously. That is why, orbit is referred as stationary shell.
6. The orbit in which an electron is permitted to move, its angular momentum is an integral multiple of h/2π
Or, mvr = nh/2π.
Electromagnetic radiation or wave theory.
This theory was developed by J. C. Maxwell. According to this theory-
1. The emission of energy from any source is continuous and wave like hence this emission is called wave or radiation.
2. Each radition consists of oscillating electric and magnetic field which are perpendicular to each other and both are perpendicular to the direction of propagation of the wave or radiation.
3. Unlike sound waves, electromagnetic waves don’t require medium and can move in vacuum.
4. The radiation possess wave character and travels with the velocity of light.
Some important characteristics of a wave:
Wavelength. The distance between any two consecutive crests or troughs is called wavelength. It is represented by λ (lambda).
Frequency. The number of waves passing through a point in one second is called frequency. It is represented by ν (nu). Its unit is Hertz (Hz).
Amplitude. The height of the Crest or the depth of the trough is called amplitude. It is represented by ‘a’. It is expressed in the units of wavelength.
Wave number. The number of waves present in 1 cm length. It is equal to the reciprocal of wavelength. It is represented by ū (nu bar).
Velocity. The linear distance travelled by the wave in one second is called velocity of wave. It is represented by ‘c’. Where c = 3.0 × 108 m/s.
Relation between velocity, wavelength and frequency of a wave: c = v × λ
Electromagnetic spectrum. The different types of electromagnetic radiation differ only in their wavelengths and frequencies. Their wavelengths increase in the following order.
Cosmic rays < y – rays < X – rays < Ultraviolet rays < visible light < Infrared rays < Microwaves < Radio waves.
Limitations of electromagnetic wave theory.
Electromagnetic wave theory was successful in explaining the properties of light such as interference, diffraction etc. But it could not explain the following:
1. The phenomenon of black body radiation.
2. The photoelectric effect
3. The radiation of heat capacity of solids as a function of temperature.
4. The line spectra of atoms with special reference to hydrogen.
Black body radiation. If the substance being heated is a black body and the radiation emitted is called black body radiation.
According to the electromagnetic wave theory, the energy is emitted or absorbed continuously. Hence, the energy of any electromagnetic radiation is proportional to its intensity and is independent of its frequency or wavelength. Hence, the body being heated should have the same Colour, although its intensity may vary as the heating is continued. But practically result is different.
Photoelectric effect. When radiation with certain frequency, strike the surface of a metal, the electrons are ejected from the surface of metal. This phenomenon is called photoelectric effect.
Work function. The minimum energy required to eject the electron is called work function. The energy of work function is called Threshold Frequency.
Planck’s quantum theory.
The main points of this theory are as follows:-
1. The radiant energy is emitted or absorbed dis continuously in the form of discrete packets of energy. Each such pocket of energy is called a ‘quantum’ and in case of light ‘photon’.
2. The energy of each quantum is directly proportional to the frequency of the radiation. E = hv.
3. The total amount of energy emitted or absorbed by a body will be some whole number quanta.
Hence, E = nhv. Where n is any integer.
Louis de Broglie wave equation.
According to this theory, all material particles possess dual characters as wave like and particle like. The wave associated with such like particles is called a matter wave or de Broglie wave.
The wave length of the wave of any material particle is equal to h/p or h/mv.
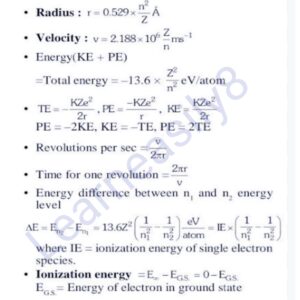
Important Formulas to be used in Atomic Structure
Heisenberg’s uncertainty principle.
According to this theory, it is impossible to measure the position and momentum of a small particle with absolute accuracy. If an attempt is made to measure any of the two quantities with higher accuracy, the other becomes less accurate. The product of the uncertainty in the position (Δx) and the uncertainty in the momentum (Δp) is equal to or greater than h/4π.
Hence, Δx . Δp > h/4π.
Aufbau principle in Atomic Structure
This principle states as follows:-
In the ground state of the atoms, the orbitals are filled in order of their increasing energies. In other words, electrons first occupy the lowest energy orbital available to them and enter into higher energy orbitals only when the lower energy orbitals are filled.
The order in which the energies of the orbitals increase and hence the order in which the orbitals are filled is as follows:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p ……
Pauli Exclusion Principle in Atomic Structure.
An orbital can have maximum two electrons and these must have opposite spin.
In other words, No two electrons of an element may have identical set of all the four quantum numbers.
Hunds rule in Atomic Structure
Electron pairing in s, p, d and f orbitals cannot occur until each orbitals of a given subshell contains one electron each or is singly occupied.
Quantum numbers. Those numbers which give complete information about all the electrons present in an atom as location, energy, the type of orbital occupied, shape and orientation of the orbitals are called quantum numbers.
There are mainly four quantum numbers which are briefly discussed below:
1. Principal quantum number. This quantum number represents the number of shells in which electrons are present. This number is denoted by ‘n’ and n = 1, 2, 3, 4 —- or K, L, M, N —– or E1, E2, E3, E4 —— etc.
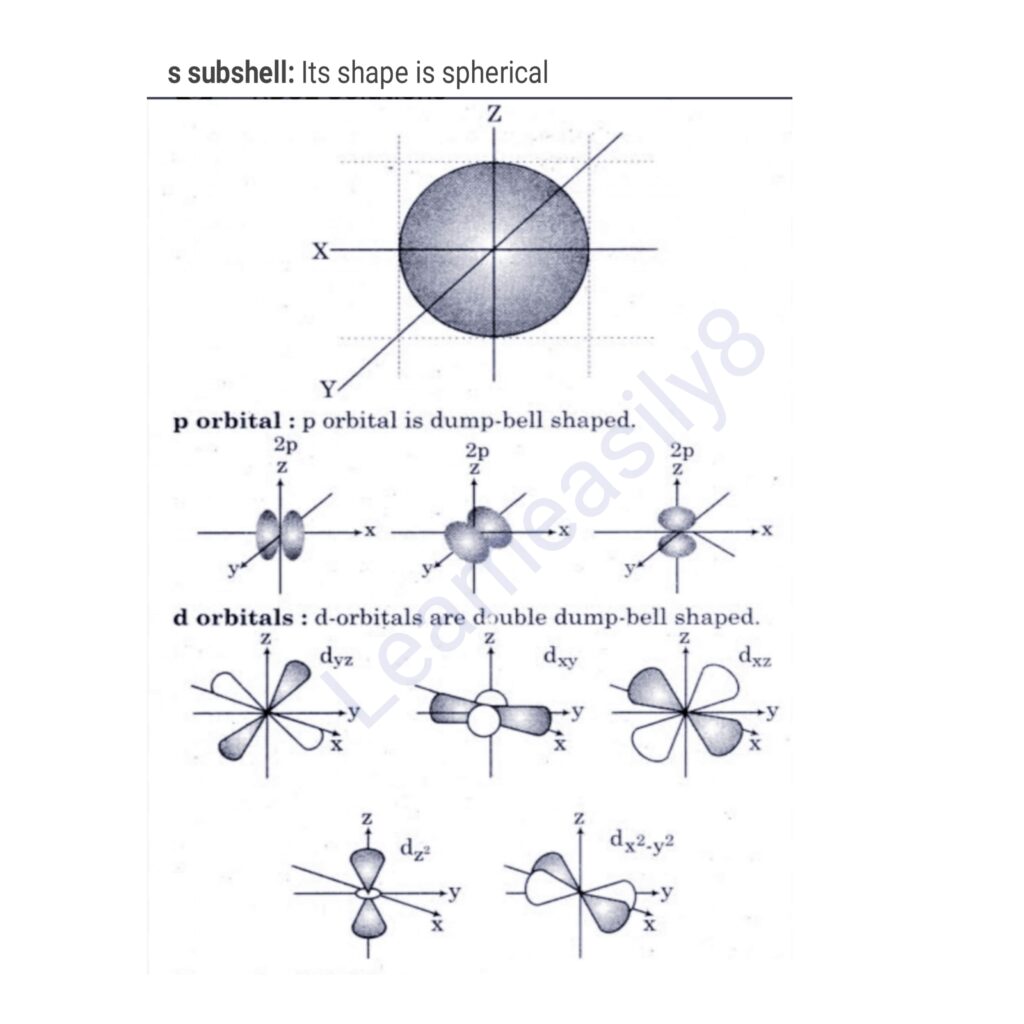
2. Azimuthal quantum number. This number indicates the subshells in which an electron is present. The subshells are s, p, d and f. This number is denoted by ‘l’ and its value depends on ‘n’. Where l = 0 to n-1.
For s-subshell, l = 0
For p-subshell, l = 1
For d-subshell, l = 2
3. Magnetic quantum number. This number represents the orientation of subshells in three dimensional space and each orientation is called orbital. This number is denoted by ‘m’ and m = – l to 0 to + l. For example if l = 2 (d-subshell), this subshell has 5 orbitals and their values are -2, -1, 0, +1, +2.
4. Spin quantum number. This number gives the information about the direction of spinning of the electron present in any orbital. It is denoted by ‘S’. S = +1/2 or -1/2.
Hydrogen Spectrum in Atomic Structure
When hydrogen gas is taken in a discharge tube and high voltage of electricity is passed through this tube. Electrons of hydrogen gas get exited and jump in higher shells. Which these electrons come back in lower shells. They release energy of various frequencies. The line Spectrum obtained w.r.t. these frequencies are called hydrogen spectrum.
There are five sets of spectral lines in hydrogen Spectrum.
1. Lyman series (n1 = 1, n2 = 2, 3, 4 —-)
2. Balmer series (n1 = 2, n2 = 3, 4, 5 —-)
3. Paschen series (n1 = 3, n2 = 4, 5, 6 —–)
4. Bracket series (n1 = 4, n2 = 5, 6, 7 —–)
5. Pfund series (n1 = 5, n2 = 6, 7, 8 —–).