There are various reactions in organic chemistry. Here we shall know about All Important Named Reactions. These reactions are important for class 11th and 12th. In this notes we shall learn all the named reactions which are always asked in the entrance exams of Neet, Jee(mains), IIT, CBSE 12th board exams and state level 12th board exams. All the reactions have been written in simple and easy language.
Any student can easily understand and remember these reactions. If these reactions are taken in memory, various types of reactions can be solved like conversions in reactions, distinction in organic compounds, reasonable questions and so on. Organic reactions are the life of organic chemistry. Let’s start to learn each reaction one by one.
All Important Named Reactions Pdf Download
All Important Named Reactions in Chemistry
All important named reactions for Class 11th
1. Birch Reduction.
Reduction of unsaturated compounds (alkenes, alkynes, and arenes) with active metals such as Li, Na or K in liquid ammonia is called Birch Reduction. This reduction is stereo selective and gives only trans- addition product. 
2. Corey – House reaction. It is an excellent reaction for the synthesis of unsymmetrical higher alkanes. In this reaction, an alkyl halide is first reacted with Lithium metal in dry Ether to form alkyl lithium which then reacts with cuprous iodide to form lithium dialkylcuprate. Which further reacts with the same or different alkyl halide and gives the corresponding symmetrical or unsymmetrical alkane. 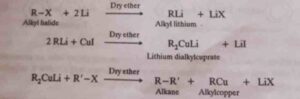
3. Diels-Alder reaction. It involves the addition of a conjugated diene (4π-electron system) to an alkene or alkyne containing an electron withdrawing group (2π-electron system) to form six membered cyclic alkenes. This reaction is commonly referred to as [4 + 2] cycloaddition reaction. 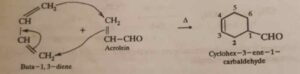
4. Fitting reaction. This is a variation of Wurtz reaction. In this reaction, two molecules of an aryl halide reacts with sodium metal in presence of dry ether to form a diaryl.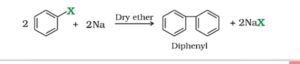
5. Friedel- Crafts alkylation. Benzene and other aromatic compounds react with alkyl halides in the presence of anhydrous aluminium chloride to form alkyl benzene. 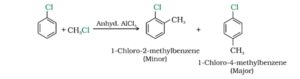
6. Friedel- Crafts acylation. Benzene and other aromatic compounds react with acid chloride or anhydrides in the presence of anhydrous aluminium chloride to form ketones.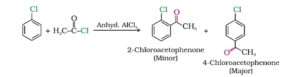
7. Kolbe’s Electrolysis. This reaction is used to prepare alkanes, alkenes and alkynes by electrolysis of aqueous solution of sodium or potassium salt of suitable acid.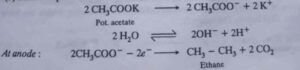
8. Sabatier – Senderens reduction. This is the reaction in which unsaturated hydrocarbons are converted into corresponding saturated hydrocarbons with hydrogen in presence of nickel as catalyst at 523 – 573K is called Sabatier – Senderens reduction.
9. Wurtz reaction. In this reaction two molecules of an alkyl halide are reacted with sodium metal in presence of dry ether to form symmetrical alkanes containing double the number of carbon atoms present in the alkyl halide.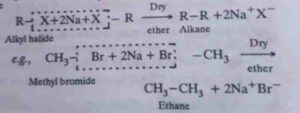
10. Wurtz – Fitting reaction. This reaction is also a variation of Wurtz reaction. In this reaction, a mixture of an aryl halide and an alkyl halide is warmed with metallic sodium in presence of dry ether for formation of homlogues of benzene.
11. Swarts reaction. It is a halogen exchange reaction. In this reaction alkyl fluoride is prepared by heating the corresponding alkyl chlorides with suitable inorganic fluorides such as AsF3, SbF3, AgF, Hg2F2 etc. 2CH3 CH2Cl + Hg2F2 → 2CH3 CH2Cl + Hg2Cl2
All important named reactions for Class 12th
12. Acylation. The replacement of an active hydrogen of alcohols, phenols or amines with an acyl (RCO or ArCO) group to form the corresponding esters or amides is called Acylation. It can be acetylation or benzonylation.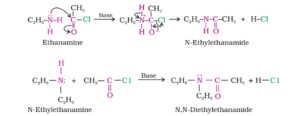
13. Aldol condensation. When two molecules of aldehydes or ketones containing α – hydrogen atoms on treatment with a dilute base (dil. NaOH, Na2CO3, Ba(OH)2 etc) undergo condensation to form β- hydroxy aldehydes or ketones is called aldol condensation.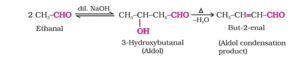
Cross aldol condensation. Aldol condensation between two different molecules of aldehydes and /or ketones is called cross aldol condensation.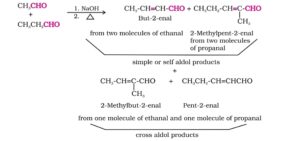
14. Balzac- Schiemann reaction. In this reaction, the aromatic primary amine is first diazotised with NaNO2 in presence of HBH4 (fluroboric acid) at 273 – 278K and the aryl diazonium tetrafluoroborate is obtained which further heated to aryl fluoride. This reaction is called Balzac- Schiemann reaction.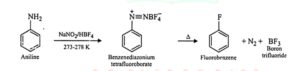
15. Benzoin condensation. Two molecules of aromatic aldehydes on heating with an ethanolic solution of KCN, undergo condensation to form benzoins. This reaction is called Benzoin condensation.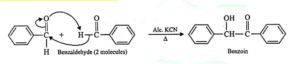
16. Bouveault – Blanc reaction. The reduction of aldehydes, ketones or esters by mean of sodium and alcohol or 1- butanol is called Bouveault – Blanc reaction.
17. Cannizzaro reaction. Aldehydes which do not contain an α- hydrogen atom, when treated with concentrated alkali solution undergo disproportionation or self oxidation – reduction reaction. As a result, one molecule of the aldehyde is reduced to the corresponding alcohol and the other molecule is oxidised to the corresponding Carboxylic acid. This reaction is called Cannizzaro reaction. It can be also either self or cross depends on the types of molecules of aldehydes.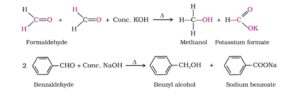
18. Carbylamine reaction. When a primary aliphatic or aromatic amine is treated with chloroform and alcoholic KOH, it forms an isocynide or carbylamine having offensive smell. This reaction is called Carbylamine reaction. CH3CH2NH2 + CHCl3 + 3KOH → CH3CH2NC + 3KCl + 3H2O
19. Claisen condensation. The self condensation of two molecules of an ester containing α- hydrogen in presence of a strong base such as sodium ethoxide to form a β – keto ester is called Claisen condensation.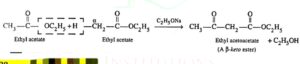
20. Claisen- Schmidt or Claisen reaction. This reaction involves the condensation between an aromatic aldehyde or a ketone with an aliphatic aldehyde or ketone in presence of dilute alkali to form α,β- unsaturated compounds.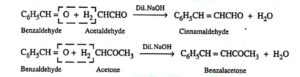
21. Clemmenson reduction. The reduction of aldehydes or ketones to the corresponding hydrocarbons with amalgamated zinc and concentrated hydrochloric acid is called Clemmenson reduction.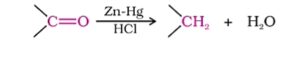
22. Coupling reaction. The reaction of diazonium salt with Phenol or aromatic amine to form azo compounds of the general formula Ar – N = N – Ar is called coupling reaction. The coupling with Phenol takes place in mildly alkaline medium while with amines it occurs under fairly acidic conditions.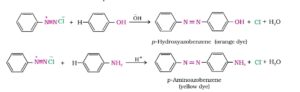
23. Decarboxylation. The process of removal of a molecule of CO2 from a Carboxylic acid is called Decarboxylation. This reaction is usually carried out by heating a Carboxylic acid or its sodium salt with soda lime (NaOH + CaO) at 630K.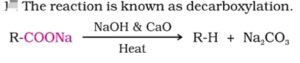
24. Diazotisation reaction. When a cold solution of a primary aromatic amine in a dilute mineral acid (HCl or H2SO4) is treated with a cold solution of nitrous acid at 273 – 278)K, arenediazonium salt is formed. This reaction is called Diazotisation reaction.
25. Esterification reaction. Alcohols react with Carboxylic acids in presence of dry HCl gas or a few drops of conc. H2SO4 as catalyst to form ester. This reaction is called Esterification reaction or Fischer Esterification.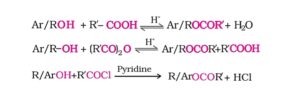
26. Etatd reaction. The oxidation of toluene with chromyl chloride (CrO2Cl2) in CCl4 or CS2 to give benzaldehyde is called Etatd reaction. In this reaction, the chromyl chloride first form a brown complex with toluene which is separated and then decomposed with dilute mineral acids to give benzaldehyde.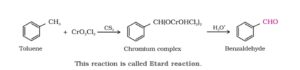
27. Finkelstein reaction. In this reaction Iodoalkane can be easily prepared from the corresponding chloro- or bromoalkanes by treating with sodium iodide in acetone or methanol. This reaction is called Finkelstein reaction.
28. Fries rearrangement. Phenyl esters on heating with anhydrous AlCl3 in presence of CS2 as solvent undergo a rearrangement in which the acyl group migrates from the phenolic oxygen atom to the o- and p- positions of the benzene ring to give a mixture of o- and p- hydroxy ketones. This reaction is called Fries rearrangement.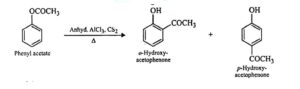
29. Gabriel phthalimde synthesis. In this reaction, phthalimde is converted into its potassium salt by treating it with alcoholic KOH. Then potassium phthalimde is heated with an alkyl halide to yield an N- alkyl phthalimide which is hydrolysed to phthalic acid and a primary amine by heating with HCl or KOH solution.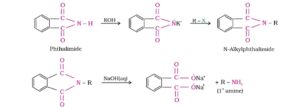
30. Gattermann reaction. This is a modification of Sandmeyer reaction in which benzene diazonium chloride is treated with Copper powder and a halogen acid to form aryl halide.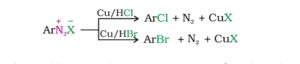
31. Gattermann- Koch reaction. When a mixture of CO and HCl gas is passed through benzene at 323K in presence of a catalyst consisting AlCl3 and a small amount of CuCl, benzaldehyde is formed. This reaction is called Gattermann- Koch reaction.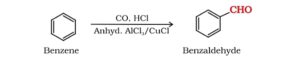
32. Grignard Synthesis. Alkyl or aryl halide reacts with magnesium turnings in presence of dry ether to form alkyl or aryl magnesium halides. This process is called Grignard Synthesis.
R – X + Mg → RMgX ( where X = Cl or Br)
33. Haloform reaction. All compounds containing the group CH3CHOH-(methyl carbinol linked to either H or carbon in alcohols or CH3CO- (methyl ketone) in an aldehyde or ketone when treated with a halogen and excess of alkali form haloform (CHX3). This reaction is called Haloform reaction. If the halogen used is iodine, yellow ppt of iodoform is formed and the reaction is called iodoform reaction.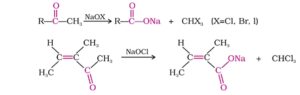
34. Hell-Volhard Zelinsky reaction. The reaction of an aliphatic Carboxylic acid containing α- hydrogens with Cl2, or Br2 in presence of a small amount of red Phosphorous to give α-haloacids is called Hell-Volhard Zelinsky reaction. With excess of halogen, all the α- hydrogen atoms of an aliphatic Carboxylic acid are replaced by halogen atoms.
35. Hofmann mustard oil reaction. When the mixture of a primary aliphatic amine, carbon disulphide and mercuric chloride is heated, alkyl isothiocyanate having a characteristic smell like that of mustard oil is formed. This reaction is called Hofmann mustard oil reaction.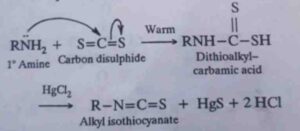
36. Hofmann bromamide degradation reaction. The conversion of a primary amide to a primary amine containing one carbon atom less than the original amide on heating with a mixture of Br2 in presence of NaOH or KOH is called Hofmann bromamide degradation reaction.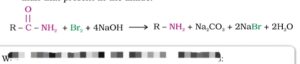
37. Hunsdiecker reaction. The decomposition of the Silver salt of a Carboxylic acid with Br2 in refluxing CCl4 to form an alkyl or aryl bromide with one carbon less than the original acid is called Hunsdiecker reaction.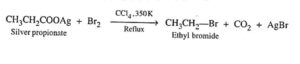
38. Kolbe reaction or Kolbe – Schmitt reaction. Sodium phenoxide reacts with carbon dioxide under pressure (4 – 7) atm at 400K to form sodium salicylate which upon acidification with mineral acids gives salicylic acid. This reaction is called Kolbe reaction.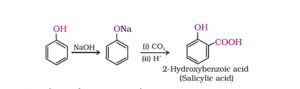
39. Liebermann’s nitroso reaction. Both aliphatic and aromatic 2o amines react with nitrous acid to give N- nitrosoamines which are generally neutral yellow oily compounds and are insoluble in dilute mineral acids. R2NH + HO – N = O → R2 – N – N = O + H2O
40. Mendius reaction. The reduction of alkyl or aryl cyanides to primary amine with nascent hydrogen produced by the action of sodium amalgam on alcohol is called Mendius reaction.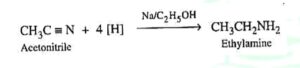
41. Perkin reaction. When an aromatic aldehyde is heated with some aliphatic acid anhydride in presence of sodium salt of the same acid, the product thus formed on acid hydrolysis gives an α,β- unsaturated acid. This reaction is called Perkin reaction.
42. Reductive amination. This means reduction in presence of ammonia. Many aldehydes and ketones react with ammonia in presence of a reducing agent such as H2/Raney Ni or NaBH3CN to form primary amines. This is called
Reductive amination.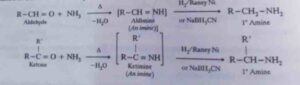
43. Reimer- Tiemann reaction. Treatment of Phenol with chloroform in presence of aqueous sodium or potassium hydroxide at 340K followed by hydrolysis of the resulting product gives 2- hydroxy benzaldehyde or salicyladehyde. This reaction is called Reimer- Tiemann reaction.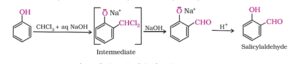
44. Rosenmund reduction. In this reaction, H2 gas is passed through boiling xylene solution of the acid chloride in presence of Pd catalyst supported over BaSO4 to obtain corresponding aldehyde and this reaction is called Rosenmund reduction.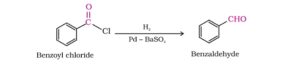
45. Sandmeyer reaction. The conversion of benzene diazonium chloride to chlorobenzene, bromo benzene or benzonitrile on treatment with CuCl/HCl or CuBr/HBr or CuCN/KCN respectively is called Sandmeyer reaction.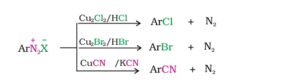
46. Schotten – Baumann reaction. The process of benzoylation of compounds containing active hydrogen such as phenol, aniline, alcohol ,etc. with benzoyl chloride in the presence of aqueous NaOH is called Schotten – Baumann reaction.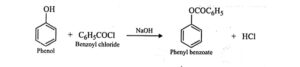
47. Stephen reaction or reduction. The partial reduction of alkyl or aryl cyanides to the corresponding aldehydes with a suspension of anhydrous stannous chloride in dry ether saturated with hydrogen chloride at room temperature followed by hydrolysis is called Stephen reaction. During this reaction, imine hydrochloride first gets precipitated which on hydrolysis with boiling water gives the corresponding aldehyde.
48. Tischenko reaction. All aldehydes with or without α- hydrogens can be made to undergo the Cannizzaro reaction on treatment with aluminium ethoxide. However, under these conditions, the alcohol and the acid produced as a result of Cannizzaro reaction, combine together to form esters. 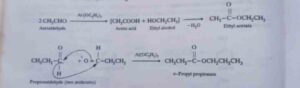
49. Transesterification. When an ester is treated with excess of another alcohol(other than the one from which the ester has been derived) in presence of a base such as sodium or potassium alkoxide or an acid H2SO4 or HCl as catalyst, a new ester and a new alcohol are formed. This alcoholysis is called Transesterification.
50. Ullmann biaryl synthesis. When iodobenzene is heated with copper powder in a sealed tube, diphenyl is produced. This reaction is called Ullmann biaryl synthesis.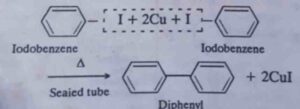
51. Williamson synthesis. In this reaction, alkyl halide reacts with sodium alkoxide or sodium phenoxide to form ethers and this reaction is called Williamson synthesis. This method is used for the preparation of both simple and mixed ethers.
R – X + R’ – O – Na → R – R’ + NaX
52. Wolff- Kishner reduction. The reduction of aldehydes and ketones to the corresponding hydrocarbons by heating them with hydrazine and KOH or potassium tert- butoxide in a high boiling solvent such as ethylene glycol is called Wolff- Kishner reduction.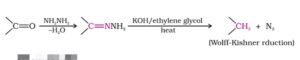
Conclusion of All important named reactions
All important named reactions have included all the important reactions which are not only specific but also referred by someone famous scientist. These reactions are distributed in many chapters. They are hydrocarbons, haloalkanes and haloarenes, alcohols, phenols, ethers, aldehydes ketones, carboxylic acids and amines. We have tried our best to bring all the named reactions at one place so that students can easily cover the all reactions in one shot. We hope that you all will have liked well after reading this article. Hence, you all are requested to share this article among your friends and favourites.
FAQ in All important named reactions
Q.No 1. What is Wurtz reaction class 12?
Ans:- In this reaction, two molecules of an alkyl halide reacts with sodium metal in presence of dry ether to form a higher alkane. This alkane has double carbon atoms that present in the given alkyl halide.
Q.No 2. How many reactions are there in organic chemistry class 12?
Ans:- There are about 35 named reactions in organic chemistry class 12th. While 15 reactions are basic like addition reaction, elimination reaction, substitution reaction, oxidation and so on.
Q.No 3. How many questions come from name reactions in NEET?
Ans:- In NEET, about 5 questions are generally asked. With the view of marks, about 20 to 24 marks can be scored from name reactions.
Q.No 4. What is the saytzeff rule class 12?
Ans:- In an elimination reaction, saytzeff rule suggests the probability of major product when reactant is an unsymmetrical alkene. According to him, the major product is that which has a greater number of alkyl groups attached to the doubly bonded carbon atoms.
Q.No 5. What is the Hoffman rule?
Ans:- This is just opposite of saytzeff rule. Hoffman suggests that the major alkene product that which is least stable and less substituted in case of elimination of fluoro compounds.

