Haloalkane and Haloarene are the important chapter for 12th board exams. This chapter is also essential for the preparation of Neet, Jee(mains) and IIT advance in organic chemistry. Haloalkanes are the halogen derivatives of alkanes. They are also known as alkyl halides. Haloarenes are the derivatives of arenes or benzene. Freon, Chloroform and DDT are very useful compounds of these families in our daily life. We shall learn very shortly and easily about these halogen compounds. We hope that you all will be sure that no questions will be left unsolved in any exams. after reading this notes.
Types of Haloalkane and Haloarene
1. Based on the number of halogen atoms, there are following types of halogen compounds.
Monohalogen compounds. Those halogen compounds in which one halogen atom is present in the chain of carbon atoms. For example, chloroethane, bromomethane, chlorobenze etc.
Dihalogen compounds. Those halogen compounds in which two halogen atoms are linked with carbon chain. In case of haloalkane, dihalogen compounds may be geminal, vicinal and poly methylene dihalide.
Poly halogen compounds. Those halogen compounds in which three or more halogen atoms are linked with carbon chain. For example Chloroform, tetrachloroethane, hexa Chlorobenzene etc.
2. Based on the hybridisation. There are following types of halogen compounds based on the types of hybridisation of carbon atom at which halogen atoms are attached.
1. Compounds containing sp3C – X bond (where X = F, Cl, Br, I)
These types of halogen compounds include alkyl halides, allylic halides and benzyl halides. These halides can be further classified as primary, secondary and tertiary halides.
2. Compounds containing sp2C – X bond.
These types of halogen compounds include aryl halide and vinyl halides. In vinyl halides, halogen atom is linked with double bonded carbon atom. In aryl halide, halogen atom is attached with the carbon atom of the ring of benzene.
3. Compounds containing spC – X bond.
In this type of haloalkane compounds, halogen atom is linked with triple bonded carbon atom. For example ethynyl chloride.
Read More: Hydrocarbons Class 11: Short and Easy Notes
Understand much more through this video.
Nature of C – X bond
- The carbon – halogen bond is polar in nature.
- Bond length of C – X bonds increases down the group from fluoride to iodide.
- Bond enthalpy of C – X bonds decreases from fluoride to iodide.
- Dipole moments of C – X have the following order: CH3Cl > CH3F > CH3Br > CH3I.
Methods for the preparation of Haloalkane and Haloarene
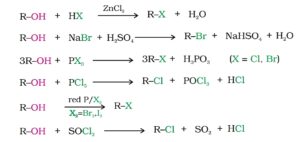
1. From Alcohols. The following reagents are used to convert alcohols into haloalkanes. They are concentrated Halogen acids, phosphorus halides or thionyl chloride. Reactions are followings:-
 2. From hydrocarbons:
2. From hydrocarbons:
⇒ By free radical halogenation:- Free radical chlorination or bromination of alkanes gives a complex mixture of isomeric mono- and polyhalo alkanes. Which is difficult to separate as pure compounds.
⇒ By electrophilic substitution:- Aryl chloride and bromide can be easily prepared by electrophilic substitu tion of chlorine and bromine respectively in the presence of Lewis acid catalysts like Iron or Iron(III).
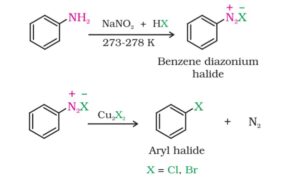
⇒ Sandmeyar’s reaction. When a primary aromatic amine is dissolved in cold aqueous mineral acid and treated with sodium nitrite, a diazonium salt is formed. Now, when this salt is mixes with cuprous chloride or cuprous bromide, Chlorobenze or bromobenze are obtained respectively. This reaction is called Sandmeyar reaction.
 ⇒ From alkenes:
⇒ From alkenes:
a. By addition of hydrogen halides: An alkene is treated with hydrogen halide (chloride, bromide or iodide) to produce corresponding alkyl halides.
⇒ In case of unsymmetrical alkenes, the products can be more than one and in that case, Markovnikov’s rule is applied. According to this rule, the major product is that which is obtained after addition of halogen atom at that carbon having more hydrogen atoms.
b. Addition of halogens: In the laboratory, addition of bromine in CCl4 to an alkene resulting in discharge of reddish brown colour of bromine and produce vic – dibromide. This is an important method for the detection of double bond in a molecule.
3. From halogen exchange:
a. By Finkelstein reaction. In this reaction, alkyl iodide is obtained after reaction between alkyl chloride or alkyl bromide with Sodium iodide in dry acetone.
b. Swarts reaction. In this reaction, alkyl chloride or bromide is heated in the presence of metallic fluoride such as AgF, Hg2F2, CoF2 or SbF3 to get alkyl fluoride.
Properties of Haloalkane and Haloarene
Physical properties of Haloalkane and Haloarene
1. Alkyl halides are colourless when it is pure. However, bromides and iodides develop colour when exposed to light.
2. Many volatile halogen compounds have sweet smell.
3. Methyl chloride, methyl bromide, ethyl chloride and some chlrofluromethane are gases. Higher members are liquids or solid at room temperature.
4. The melting and boiling point of halogen compounds is greater than alkanes having comparable molecular masses.
5. For the same alkyl group, their boiling points increase in the order: RF < RCl < RBr < RI.
6. For the same halide, the boiling point increases with increase in the size of carbon chain.
7. In case of isomeric haloalkanes, their boiling points decreases with increase in branching.
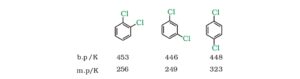
8. In case of dihalobenze, the order of melting and boiling points are followings:
9. Bromo, iodo and polychloro derivatives of hydrocarbons are heavier than water. Further, their densities increase with increase in their molecular masses.
10. The haloalkanes are very slightly soluble in water. Because less energy is released when new attraction of setup is established between haloalkanes and water molecules.
Chemical properties of Haloalkane and Haloarene
Haloalkanes are involved in the following reactions.
1. Nucleophilic substitution reactions
2. Elimination reactions
3. Reaction with metals
1. Nucleophilic substitution reactions . In this reaction, weaker nucleophile of haloalkane is replaced by stronger nucleophile. For example:
RX + CN– → RCN + X–
Types of nucleophilic substitution reactions: There are two types of nucleophilic substitution reactions.
1. SN1 reaction. It means nucleophilic substitution reaction of first order. In this reaction, the rate of reaction depends only on the concentration of substrate. The order of SN1 reaction is following.
R3C-X > R2CH-X > RCH2X > CH3X
2. SN2 reaction. It means nucleophilic substitution reaction of 2nd order. In this reaction, the rate of reaction depends on the concentration of substrate and reagent both. The order of SN2 reaction is following.
R3C-X < R2CH-X < RCH2X < CH3X.
⇒ SN1 reaction proceeds with stability of carbocations and hence forms enantiomer. While SN2 reaction proceeds with formation of unstable compound in transition state and leads to inversion of product.
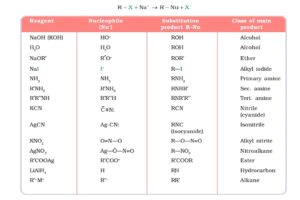
Nucleophilic substitution reactions of alkyl halides can be understood from the following charts.
2. Elimination reaction. When a haloalkane with β- hydrogen atom is heated with alcoholic solution of Potassium hydroxide, an alkene is formed after removal of hydrogen and halogen atom. This reaction is also known as β- elimination or dehydrohalogenation reaction. For example:
CH3CH2Cl + KOH(alc) → CH2 = CH2 + KCl + H2O
If there is possibility of more than one alkene due to the possibility of more than one β- hydrogen atoms. One alkene is considered as the major product. According to Saytzeff’s rule, the major product is that alkene which is most stable.
3. Reaction with metals.
- Reaction with Magnesium. When alkyl halides react with magnesium in the presence of dry ether, organometallic compounds are formed and these compounds are called Grignard reagents.
RX + Mg → RMgX (alkyl magnesium halide. - Reaction with Sodium. Alkyl halides react with sodium in dry ether to give hydrocarbons containing double the number of carbon atoms present in alkyl halides. This reaction is called Wurtz reaction.
2RX + 2Na ——- R – R + 2NaX.
Reactions of Haloarenes
1. Nucleophilic substitution reactions. Aryl halides are extremely less reactive towards nucleophilic substitution reactions due to the following reasons:
A. Resonance effect
B. Difference in hybridisation of carbon atom in C – X bond
C. Instability of phenyl carbo cation.
D. Presence of high electron density inside the benzene ring.
However, Chlorobenze can be converted into Phenol by heating in aqueous sodium hydroxide solution at a temperature of 623K and a pressure of 300 atmosphere.
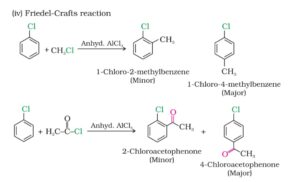
 2. Electrophilic substitution reactions. Haloarenes undergo the usual electrophilic substitution reactions such as halogenation, nitration, sulphonation and Friedel crafts reactions.
2. Electrophilic substitution reactions. Haloarenes undergo the usual electrophilic substitution reactions such as halogenation, nitration, sulphonation and Friedel crafts reactions. 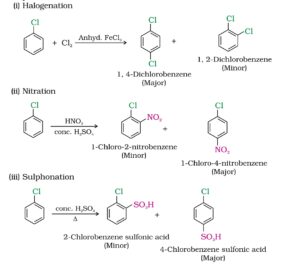
 Reaction with metals
Reaction with metals
a. Wurtz Fitting reaction. A mixture of alkyl halide and aryl halide gives an alkyl arene when treated with sodium in presence of dry ether. This reaction is called Wurtz Fitting reaction.
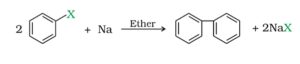
 b. Fitting reaction. Aryl halides when treated with sodium in presence of dry ether, it produces biphenyl. This reaction is called Fitting reaction.
b. Fitting reaction. Aryl halides when treated with sodium in presence of dry ether, it produces biphenyl. This reaction is called Fitting reaction.
 Summary of Haloalkane and Haloarene
Summary of Haloalkane and Haloarene
In this article or notes, we have discussed all the important topics. Kinds of Haloalkane and Haloarene have been discussed properly. The nature of halogen compounds, important methods of preparation of all the types of Haloalkane and Haloarene have been detailed with examples. Physical properties and chemical properties of both Haloalkane and Haloarene have been written in suitable manners.
We have also discussed types of nucleophilic substitution reactions and their order of reactivity. We hope that this article will be very beneficial to all the students who are preparing for board exams for 12th or other entrance exams like jee mains, IIT advance or neet. Therefore, we shall request to share this article among your friends and favorites. Thanks for reading this article.
FAQS of Haloalkane and Haloarene
Q.No 1. What are called Freons?
Ans chlorofluoro compounds of methane and ethane in which all the H-atoms are replaced by halogen atoms are called Freons. For example CF4, CF2Cl2, CF3Cl etc.
Q.No 2. What is the full form of DDT?
The full form of DDT is p,p’ – Dichlorodiphenyltrichloroethane.
Q.No 3. What is the use of idoform?
Ans Idoform (CHI3) was earlier used as an antiseptic for dressing wounds due to the liberation of Iodine.
Q.No 4. What are the difference between retention and inversion?
Ans If the relative configuration of the atoms/groups around a chiral centre in an optically active molecule remains the same before and after the reaction the reaction is said to proceed with retention of configuration. On the other hand the relative configuration of the atoms or groups around a streocentre in the product is opposite to that in the reactant, the reaction is said to proceed with inversion of configuration.

